DNA damage tolerance in stem cells, ageing, mutagenesis, disease and cancer therapy
- PMID: 31251805
- PMCID: PMC6698745
- DOI: 10.1093/nar/gkz531
DNA damage tolerance in stem cells, ageing, mutagenesis, disease and cancer therapy
Abstract
The DNA damage response network guards the stability of the genome from a plethora of exogenous and endogenous insults. An essential feature of the DNA damage response network is its capacity to tolerate DNA damage and structural impediments during DNA synthesis. This capacity, referred to as DNA damage tolerance (DDT), contributes to replication fork progression and stability in the presence of blocking structures or DNA lesions. Defective DDT can lead to a prolonged fork arrest and eventually cumulate in a fork collapse that involves the formation of DNA double strand breaks. Four principal modes of DDT have been distinguished: translesion synthesis, fork reversal, template switching and repriming. All DDT modes warrant continuation of replication through bypassing the fork stalling impediment or repriming downstream of the impediment in combination with filling of the single-stranded DNA gaps. In this way, DDT prevents secondary DNA damage and critically contributes to genome stability and cellular fitness. DDT plays a key role in mutagenesis, stem cell maintenance, ageing and the prevention of cancer. This review provides an overview of the role of DDT in these aspects.
© The Author(s) 2019. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures
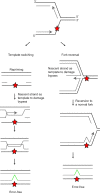
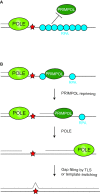
Similar articles
-
DNA damage tolerance in hematopoietic stem and progenitor cells in mice.Proc Natl Acad Sci U S A. 2017 Aug 15;114(33):E6875-E6883. doi: 10.1073/pnas.1706508114. Epub 2017 Jul 31. Proc Natl Acad Sci U S A. 2017. PMID: 28761001 Free PMC article.
-
DNA Damage Tolerance Pathways in Human Cells: A Potential Therapeutic Target.Front Oncol. 2022 Feb 7;11:822500. doi: 10.3389/fonc.2021.822500. eCollection 2021. Front Oncol. 2022. PMID: 35198436 Free PMC article. Review.
-
Repriming DNA synthesis: an intrinsic restart pathway that maintains efficient genome replication.Nucleic Acids Res. 2021 May 21;49(9):4831-4847. doi: 10.1093/nar/gkab176. Nucleic Acids Res. 2021. PMID: 33744934 Free PMC article. Review.
-
Implications of Translesion DNA Synthesis Polymerases on Genomic Stability and Human Health.Mol Cell Biol. 2023;43(8):401-425. doi: 10.1080/10985549.2023.2224199. Epub 2023 Jul 13. Mol Cell Biol. 2023. PMID: 37439479 Free PMC article.
-
Post-Translational Modifications of PCNA in Control of DNA Synthesis and DNA Damage Tolerance-the Implications in Carcinogenesis.Int J Biol Sci. 2021 Sep 23;17(14):4047-4059. doi: 10.7150/ijbs.64628. eCollection 2021. Int J Biol Sci. 2021. PMID: 34671219 Free PMC article. Review.
Cited by
-
Rad1 attenuates DNA double-strand breaks and cell cycle arrest in type II alveolar epithelial cells of rats with bronchopulmonary dysplasia.Mol Med. 2023 May 24;29(1):70. doi: 10.1186/s10020-023-00660-3. Mol Med. 2023. PMID: 37226090 Free PMC article.
-
Saccharomyces cerevisiae Rev7 promotes non-homologous end-joining by blocking Mre11 nuclease and Rad50's ATPase activities and homologous recombination.Elife. 2024 Dec 4;13:RP96933. doi: 10.7554/eLife.96933. Elife. 2024. PMID: 39630591 Free PMC article.
-
Palindromes in DNA-A Risk for Genome Stability and Implications in Cancer.Int J Mol Sci. 2021 Mar 11;22(6):2840. doi: 10.3390/ijms22062840. Int J Mol Sci. 2021. PMID: 33799581 Free PMC article. Review.
-
Individual Genetic Heterogeneity.Genes (Basel). 2022 Sep 10;13(9):1626. doi: 10.3390/genes13091626. Genes (Basel). 2022. PMID: 36140794 Free PMC article. Review.
-
Flap endonuclease 1 and DNA-PKcs synergistically participate in stabilizing replication fork to encounter replication stress in glioma cells.J Exp Clin Cancer Res. 2022 Apr 12;41(1):140. doi: 10.1186/s13046-022-02334-0. J Exp Clin Cancer Res. 2022. PMID: 35414100 Free PMC article.
References
-
- Hoeijmakers J.H. DNA damage, aging, and cancer. N. Engl. J. Med. 2009; 361:1475–1485. - PubMed
-
- Lindahl T., Barnes D.E.. Repair of endogenous DNA damage. Cold Spring Harb. Symp. Quant. Biol. 2000; 65:127–133. - PubMed
-
- De Bont R., van Larebeke N.. Endogenous DNA damage in humans: a review of quantitative data. Mutagenesis. 2004; 19:169–185. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

