IL-4Rα Blockade by Dupilumab Decreases Staphylococcus aureus Colonization and Increases Microbial Diversity in Atopic Dermatitis
- PMID: 31252032
- PMCID: PMC7163930
- DOI: 10.1016/j.jid.2019.05.024
IL-4Rα Blockade by Dupilumab Decreases Staphylococcus aureus Colonization and Increases Microbial Diversity in Atopic Dermatitis
Abstract
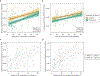
Dupilumab is a fully human antibody to interleukin-4 receptor α that improves the signs and symptoms of moderate to severe atopic dermatitis (AD). To determine the effects of dupilumab on Staphylococcus aureus colonization and microbial diversity on the skin, bacterial DNA was analyzed from swabs collected from lesional and nonlesional skin in a double-blind, placebo-controlled study of 54 patients with moderate to severe AD randomized (1:1) and treated with either dupilumab (200 mg weekly) or placebo for 16 weeks. Microbial diversity and relative abundance of Staphylococcus were assessed by DNA sequencing of 16S ribosomal RNA, and absolute S. aureus abundance was measured by quantitative PCR. Before treatment, lesional skin had lower microbial diversity and higher overall abundance of S. aureus than nonlesional skin. During dupilumab treatment, microbial diversity increased and the abundance of S. aureus decreased. Pronounced changes were seen in nonlesional and lesional skin. Decreased S. aureus abundance during dupilumab treatment correlated with clinical improvement of AD and biomarkers of type 2 immunity. We conclude that clinical improvement of AD that is mediated by interleukin-4 receptor α inhibition and the subsequent suppression of type 2 inflammation is correlated with increased microbial diversity and reduced abundance of S. aureus.
Trial registration: ClinicalTrials.gov NCT01979016.
Copyright © 2019 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
CONFLICT OF INTEREST
Outside of the submitted work, NT was an investigator for and has received research grants from Regeneron Pharmaceuticals, Inc.; RK was an scientific advisory board member and consultant for Janssen R&D LLC, Commense, Inc., and Prometheus Lab Inc.; MA, NMHG, and JH were employees and shareholders of Regeneron Pharmaceuticals, Inc.; TH and GP were employees of Sanofi and may hold stock and/or stock options in the company; EG was an investigator for AbbVie, Celgene, Eli Lilly and Company, GlaxoSmithKline, Regeneron Pharmaceuticals, Inc., Sanofi, Leo Pharma, Pfizer, Galderma, and Glenmark; served as a consultant for AbbVie, Anacor, Eli Lilly and Company, Galderma, GlaxoSmithKline, Leo Pharma, Menlo Therapeutics, Kiniksa, Pfizer, Realm, Regeneron Pharmaceuticals, Inc., Sanofi, Asana Biosciences, DBV, Kyowa, Daiichi Sankyo, Glenmark, Novartis, Dermira; and received research grants from Regeneron Pharmaceuticals, Inc., Sanofi, Pfizer, Galderma, Janssen, Celgene, Novartis, Dermira, AbbVie, Innovaderm, and Leo Pharma. RB was an investigator, consultant, advisory board member, speaker, and/or received honoraria from Antibiotx, Aquinox Pharma, Asana, Astellas, Brickell Biotech, Dermavant, Dermira, Dignity Sciences, Galderma, Glenmark, GSK-Stiefel, Hoffman-LaRoche Ltd., Kiniksa, Leo Pharma, Neokera, Pfizer, Regeneron Pharmaceuticals, Inc., Sienna Biopharmaceuticals, and Vitae Pharmaceuticals; and was a shareholder of Innovaderm Research; JIS was an investigator for AbbVie, Celgene, Chugai, Eli Lilly and Company, GlaxoSmithKline, Regeneron Pharmaceuticals, Inc., and Sanofi; a consultant for AbbVie, Anacor, Eli Lilly and Company, Galderma, GlaxoSmithKline, Incyte, Leo Pharma, Menlo, Kiniksa, Pfizer, Realm, Regeneron Pharmaceuticals, Inc., and Sanofi; and a speaker for Regeneron Pharmaceuticals, Inc., and Sanofi; JK was a consultant and recipient of research grants from Amgen, Bristol-Myers Squibb, Boehringer Ingelheim, Dermira, Innovaderm, Janssen, Kadmon, Kyowa, Eli Lilly and Company, Merck, Novartis, Parexel, and Pfizer; was consultant and received personal fees from AbbVie, Baxter, Biogen Idec, Delenex, Kineta, Sanofi, Serono, and XenoPort; and was an investigator for Regeneron Pharmaceuticals, Inc.; AM served on the advisory board and was a consultant, investigator, and speaker and received grants and honoraria from AbbVie, Amgen, Janssen Biotech, Inc., and Leo Pharma; served on the advisory board and was a consultant and investigator and received grants and honoraria from Allergan; served on the advisory board and was an investigator and recipient of grants and honoraria from Boehringer Ingelheim; served as a consultant and investigator and was a recipient of grants and honoraria from Novartis, Pfizer, and XenoPort; was investigator and recipient of grants from Anacor, Celgene, Dermira, Merck, Neothetics, Regeneron Pharmaceuticals, Inc., and Symbio/Maruho; served on the advisory board, was an consultant and investigator, and received honoraria from Eli Lilly and Company; and was consultant and received honoraria from Galderma and Vitae Pharmaceuticals; RG was an investigator and recipient of research grants from Regeneron Pharmaceuticals, Inc.; had equity interest in and was co-founder and SAB of MatriSys Bioscience; had equity interest in and was SAB of Sente, Inc.; was a consultant for Roche; received a research grant from Novan; and received a research grant from Colgate Palmolive. The other authors state no conflict of interest.
Figures





Comment in
-
Targeting T2 Inflammation by Dupilumab Impacts on the Microbiomic "Ménage à Trois" of Atopic Dermatitis.J Invest Dermatol. 2020 Jan;140(1):15-17. doi: 10.1016/j.jid.2019.07.680. J Invest Dermatol. 2020. PMID: 31864429
References
-
- Beck LA, Thaçi D, Hamilton JD, Graham NM, Bieber T, Rocklin R, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med 2014;371:130–9. - PubMed
-
- Bieber T Atopic dermatitis. N Engl J Med 2008;358:1483–94. - PubMed
-
- Bjerre RD, Bandier J, Skov L, Engstrand L, Johansen JD. The role of the skin microbiome in atopic dermatitis: a systematic review. Br J Dermatol 2017;177:1272–8. - PubMed
-
- Blauvelt A, de Bruin-Weller M, Gooderham M, Cather JC, Weisman J, Pariser D, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet 2017;389:2287–303. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

