IL-20-Receptor Signaling Delimits IL-17 Production in Psoriatic Inflammation
- PMID: 31252033
- PMCID: PMC6926146
- DOI: 10.1016/j.jid.2019.06.127
IL-20-Receptor Signaling Delimits IL-17 Production in Psoriatic Inflammation
Abstract
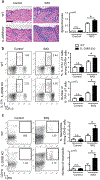
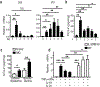
IL-17 cytokines, in particular IL-17A, are critical effectors in psoriasis. Antibodies that block IL-17A are highly efficacious in treating psoriasis. Likewise, disruption of IL-17 cytokines signaling, such as via the loss of the adaptor CIKS/Act1, ameliorates inflammation in mouse models of psoriasis. IL-17A promotes a cascade of effects, including the robust production of IL-19 in both humans and mice. IL-19, along with IL-20 and IL-24, signal via IL-20 receptors and comprise a subgroup within the IL-10 cytokine family. The role of these three cytokines in psoriasis is unresolved. They have been linked to inflammatory processes, including psoriatic pathology, but these cytokines have also been reported to suppress inflammation in other contexts. In this study, we demonstrate that signaling via IL-20 receptors, including in response to IL-19, delimited aspects of imiquimod-induced psoriatic inflammation. IL-20 receptor signaling suppressed the dermal production of the CCL2 chemokine and thereby reduced CCL-2-driven infiltration of inflammatory cells into the dermis, including IL-17A-producing γδT cells. This constitutes a negative feedback, since IL-17A strongly induces IL-19 in keratinocytes. The effects of IL-17 cytokines in this inflammatory setting are dynamic; they are central to the development of both dermal and epidermal hallmarks of psoriasis but also initiate a path to mitigate inflammatory damage.
Copyright © 2019 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
CONFLICT OF INTEREST
The authors state no conflict of interest.
Figures
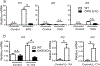



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

