Marine-Derived Natural Lead Compound Disulfide-Linked Dimer Psammaplin A: Biological Activity and Structural Modification
- PMID: 31252563
- PMCID: PMC6669562
- DOI: 10.3390/md17070384
Marine-Derived Natural Lead Compound Disulfide-Linked Dimer Psammaplin A: Biological Activity and Structural Modification
Abstract
Marine natural products are considered to be valuable resources that are furnished with diverse chemical structures and various bioactivities. To date, there are seven compounds derived from marine natural products which have been approved as therapeutic drugs by the U.S. Food and Drug Administration. Numerous bromotyrosine derivatives have been isolated as a type of marine natural products. Among them, psammaplin A, including the oxime groups and carbon-sulfur bonds, was the first identified symmetrical bromotyrosine-derived disulfide dimer. It has been found to have a broad bioactive spectrum, especially in terms of antimicrobial and antiproliferative activities. The highest potential indole-derived psammaplin A derivative, UVI5008, is used as an epigenetic modulator with multiple enzyme inhibitory activities. Inspired by these reasons, psammaplin A has gradually become a research focus for pharmacologists and chemists. To the best of our knowledge, there is no systematic review about the biological activity and structural modification of psammaplin A. In this review, the pharmacological effects, total synthesis, and synthesized derivatives of psammaplin A are summarized.
Keywords: biological activity; marine natural product; psammaplin A; structural modification.
Conflict of interest statement
The authors declare no conflict of interest.
Figures












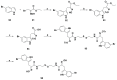





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

