Computational Investigation of Bisphosphate Inhibitors of 3-Deoxy-d- manno-octulosonate 8-phosphate Synthase
- PMID: 31252580
- PMCID: PMC6650799
- DOI: 10.3390/molecules24132370
Computational Investigation of Bisphosphate Inhibitors of 3-Deoxy-d- manno-octulosonate 8-phosphate Synthase
Abstract
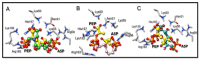
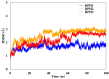
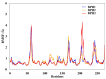
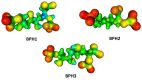
The synthase, 3-deoxy-d-manno-octulosonate 8-phosphate (KDO8P), is a key enzyme for the lipopolysaccharide (LPS) biosynthesis of gram-negative bacteria and a potential target for developing new antimicrobial agents. In this study, computational molecular modeling methods were used to determine the complete structure of the KDO8P synthase from Neisseria meningitidis and to investigate the molecular mechanism of its inhibition by three bisphosphate inhibitors: BPH1, BPH2, and BPH3. Our results showed that BPH1 presented a protein-ligand complex with the highest affinity, which is in agreement with experimental data. Furthermore, molecular dynamics (MD) simulations showed that BPH1 is more active due to the many effective interactions, most of which are derived from its phosphoenolpyruvate moiety. Conversely, BPH2 exhibited few hydrogen interactions during the MD simulations with key residues located at the active sites of the KDO8P synthase. In addition, we hydroxylated BPH2 to create the hypothetical molecule named BPH3, to investigate the influence of the hydroxyl groups on the affinity of the bisphosphate inhibitors toward the KDO8P synthase. Overall, we discuss the main interactions between the KDO8P synthase and the bisphosphate inhibitors that are potential starting points for the design of new molecules with significant antibiotic activities.
Keywords: KDO8P synthase; Neisseria meningitidis; bisphosphate inhibitors; molecular dynamics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

