Evaluation of systemic absorption and bronchodilator effect of glycopyrronium bromide delivered by nebulizer or a dry powder inhaler in subjects with chronic obstructive pulmonary disease
- PMID: 31253162
- PMCID: PMC6599298
- DOI: 10.1186/s12931-019-1113-z
Evaluation of systemic absorption and bronchodilator effect of glycopyrronium bromide delivered by nebulizer or a dry powder inhaler in subjects with chronic obstructive pulmonary disease
Abstract
Background: Effective bronchodilator therapy depends upon adequate drug deposition in the lung. COPD patients who are unable to administer medications efficiently with conventional inhalers may benefit from the use of a nebulizer device. The aim of this study was to evaluate the systemic bioavailability and bronchodilator response of glycopyrronium bromide (GLY) administered by a novel nebulizer (eFlow® closed system [CS] vibrating membrane nebulizer) or dry powder inhaler (DPI) in subjects with moderate-to-severe chronic obstructive pulmonary disease (COPD).
Methods: In this randomized, open-label, single-dose, five-way crossover study, subjects received a sequence of either 50 μg GLY delivered by eFlow CS nebulizer (GLY/eFlow) or 63 μg GLY delivered by DPI (GLY/DPI), with and without activated charcoal, followed by intravenous infusion of 50 μg GLY with a washout period of 7 days between doses. Endpoints included plasma pharmacokinetics, safety and efficacy.
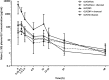
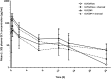
Results: The mean (± SD) baseline predicted forced expiratory volume in 1 s (FEV1) of the 30 subjects who completed the study was 51 ± 15%, with a FEV1/forced vital capacity ratio of 50 ± 11%. Without charcoal, the absolute systemic bioavailability of GLY/eFlow and GLY/DPI were approximately 15 and 22%, respectively. Changes from baseline in FEV1 at 60 min post-dose, without administration of charcoal, were 0.180 L and 0.220 L for GLY/eFlow and GLY/DPI, respectively; FEV1 improvements were similar when charcoal was administered (0.220 L for both GLY/eFlow and GLY/DPI). There were no significant differences in spirometry between the two devices. Fewer subjects administered GLY/eFlow reported adverse events (n = 15) than GLY/DPI (n = 18).
Conclusions: After single doses, GLY/DPI delivered numerically higher peak and steady state levels of drug than did GLY/eFlow. Nebulized GLY produced similar bronchodilation but lower systemic levels of drug than GLY/DPI. Slightly higher number of subjects reported adverse events with GLY/DPI than with GLY/eFlow. Nebulized GLY may offer an effective alternative to patients with COPD not adequately treated with other devices.
Trial registration: NCT02512302 (ClinicalTrials.gov). Registered 28 May 2015.
Keywords: Bioavailability; COPD; Glycopyrronium bromide; Nebulizer.
Conflict of interest statement
TG, AO-G and GG are employees of Sunovion Pharmaceuticals Inc.
Figures



Similar articles
-
Satisfaction with the Use of eFlow Closed-System Nebulizer in Patients with Moderate-to-Very Severe Chronic Obstructive Pulmonary Disease: Findings from a Long-Term Safety Study.J Aerosol Med Pulm Drug Deliv. 2019 Feb;32(1):24-33. doi: 10.1089/jamp.2018.1477. Epub 2018 Nov 17. J Aerosol Med Pulm Drug Deliv. 2019. PMID: 30457433 Clinical Trial.
-
Long-term safety of glycopyrrolate/eFlow® CS in moderate-to-very-severe COPD: Results from the Glycopyrrolate for Obstructive Lung Disease via Electronic Nebulizer (GOLDEN) 5 randomized study.Respir Med. 2017 Nov;132:251-260. doi: 10.1016/j.rmed.2017.08.020. Epub 2017 Aug 24. Respir Med. 2017. PMID: 28919143 Clinical Trial.
-
Efficacy and safety of glycopyrrolate/eFlow® CS (nebulized glycopyrrolate) in moderate-to-very-severe COPD: Results from the glycopyrrolate for obstructive lung disease via electronic nebulizer (GOLDEN) 3 and 4 randomized controlled trials.Respir Med. 2017 Nov;132:238-250. doi: 10.1016/j.rmed.2017.07.011. Epub 2017 Jul 19. Respir Med. 2017. PMID: 28838685 Clinical Trial.
-
Glycopyrrolate/eFlow CS: The First Nebulized Long-Acting Muscarinic Antagonist Approved to Treat Chronic Obstructive Pulmonary Disease.Ann Pharmacother. 2019 Mar;53(3):285-293. doi: 10.1177/1060028018798753. Epub 2018 Sep 1. Ann Pharmacother. 2019. PMID: 30175596 Free PMC article. Review.
-
An overview of glycopyrrolate/eFlow® CS in COPD.Expert Rev Respir Med. 2018 Jun;12(6):447-459. doi: 10.1080/17476348.2018.1476853. Epub 2018 May 28. Expert Rev Respir Med. 2018. PMID: 29774778 Review.
Cited by
-
Inhalable microparticles as drug delivery systems to the lungs in a dry powder formulations.Regen Biomater. 2022 Dec 8;10:rbac099. doi: 10.1093/rb/rbac099. eCollection 2023. Regen Biomater. 2022. PMID: 36683752 Free PMC article. Review.
-
Future Trends in Nebulized Therapies for Pulmonary Disease.J Pers Med. 2020 May 10;10(2):37. doi: 10.3390/jpm10020037. J Pers Med. 2020. PMID: 32397615 Free PMC article. Review.
References
-
- Sunovion Pharmaceuticals Inc . LONHALA® MAGNAIR® prescribing information. 2018.
-
- Melani AS, Bonavia M, Cilenti V, Cinti C, Lodi M, Martucci P, Serra M, Scichilone N, Sestini P, Aliani M, Neri M, Gruppo Educazionale Associazione Italiana Pneumologi Ospedalieri Inhaler mishandling remains common in real life and is associated with reduced disease control. Respir Med. 2011;105:930–938. doi: 10.1016/j.rmed.2011.01.005. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

