Interactions of Rabconnectin-3 with Cav2 calcium channels
- PMID: 31253182
- PMCID: PMC6599304
- DOI: 10.1186/s13041-019-0483-y
Interactions of Rabconnectin-3 with Cav2 calcium channels
Abstract
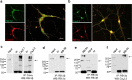
This study describes the interaction between Cav2 calcium channels and Rabconnectin-3, a di-subunit protein that is associated with synaptic vesicles. Immunostaining reveals that both Rabconnectin-3α (RB-3α) and Rabconnectin-3β (RB-3β) are colocalized in mouse hippocampal neurons. Co-immunoprecipitations from brain tissue is consistent with the formation of a protein complex between RB-3α and RB-3β and both Cav2.2 and the related Cav2.1 calcium channel. The coexpression of either RB-3α or RB-3β with Cav2.2 calcium channels in tsA-201 cells led to a reduction in Cav2.2 current density without any effects on the voltage-dependence of activation or inactivation. Coexpression of both Rabconnectin-3 subunits did not cause an additive effect on current densities. Finally, the presence of Rabconnectin-3 did not interfere with μ-opioid receptor mediated Gβγ modulation of Cav2.2 channels. Altogether, our findings show that Rabconnectin-3 has the propensity to regulate calcium entry mediated by Cav2.2 channels.
Keywords: Cav2,2 calcium channels; Hippocampus; N-type channels; Opioid receptor; Rabconnectin-3.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

