Total laparoscopic and thoracoscopic Ivor Lewis esophagectomy after neoadjuvant Chemoradiation with minimal overall and anastomotic complications
- PMID: 31253184
- PMCID: PMC6599249
- DOI: 10.1186/s13019-019-0937-4
Total laparoscopic and thoracoscopic Ivor Lewis esophagectomy after neoadjuvant Chemoradiation with minimal overall and anastomotic complications
Abstract
Background: The published rates of morbidity and mortality remain relatively high for patients who undergo laparoscopic and thoracoscopic Ivor Lewis esophagectomy. We report the postoperative and oncologic outcomes of a large cohort of patients with esophageal carcinoma who were uniformly treated with laparoscopic and thoracoscopic Ivor Lewis esophagectomy following neoadjuvant chemoradiation.
Methods: This is a retrospective observational study of 112 patients diagnosed with esophageal carcinoma who underwent total laparoscopic and thoracoscopic Ivor Lewis esophagectomy from May 2014 to May 2018. All of the patients received neoadjuvant chemoradiation consisting of 45 to 50.4 Gray of radiation and 3-5 cycles of carboplatin and paclitaxel chemotherapy. Perioperative morbidity and 90-day mortality were recorded. The overall and disease-free survival rates were estimated by Kaplan Meier techniques.
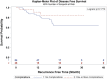
Results: A total of 112 patients completed induction chemoradiation followed by a total laparoscopic and thoracoscopic Ivor Lewis esophagectomy. There were 87 (77.68%) males and 25 (22.32%) females with a mean age of 61.6 years ± 10.4. A total of 28 (25%) patients had one or more complications. A total of 4 patients (3.57%) had an anastomotic leak. The 90-day mortality rate was 0.89%. The 3-year overall survival rate was 64.7% and the 3-year disease-free survival rate was 70.2%.
Conclusion: The current outcomes suggest that laparoscopic and thoracoscopic Ivor Lewis esophagectomy can be performed with minimal overall and anastomotic complications following neoadjuvant chemoradiation.
Keywords: Esophageal Cancer; Induction therapy; Minimally invasive Esophagectomy.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
-
- Howlander N, Noone AM, Krapcho M, et al. National Cancer Institute. SEER Cancer Statistics Review, 1975–2015. http://seer.cancer.gov/csr/1975_2016/, based on November 2017 SEER data submission, posted to the SEER web site, April 2018.
-
- Van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:20174–20184. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

