The mitochondrial calcium uniporter contributes to morphine tolerance through pCREB and CPEB1 in rat spinal cord dorsal horn
- PMID: 31253357
- PMCID: PMC6676161
- DOI: 10.1016/j.bja.2019.05.027
The mitochondrial calcium uniporter contributes to morphine tolerance through pCREB and CPEB1 in rat spinal cord dorsal horn
Abstract
Background: The long-term use of opioid analgesics is limited by the development of unwanted side-effects, such as tolerance. The molecular mechanisms of morphine anti-nociceptive tolerance are still unclear. The mitochondrial calcium uniporter (MCU) is involved in painful hyperalgesia, but the role of MCU in morphine tolerance has not been uncharacterised.
Methods: Rats received intrathecal injection of morphine for 7 days to induce morphine tolerance. The mechanical withdrawal threshold was measured using von Frey filaments, and thermal latency using the hotplate test. The effects of an MCU inhibitor, antisense oligodeoxynucleotide against cyclic adenosine monophosphate response element (CRE)-binding protein (CREB) or cytoplasmic polyadenylation element-binding protein 1 (CPEB1) in morphine tolerance were examined.
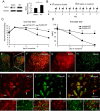
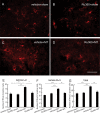
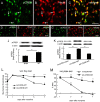
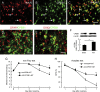
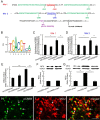
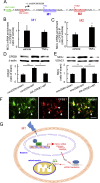
Results: Spinal morphine tolerance was associated with an increased expression of neuronal MCU, phospho-CREB (pCREB), and CPEB1 in the spinal cord dorsal horn. MCU inhibition increased the mechanical threshold and thermal latency, and reduced the accumulation of mitochondrial calcium in morphine tolerance. Intrathecal antisense oligodeoxynucleotide against CREB or CPEB1 restored the anti-nociceptive effects of morphine compared with mismatch oligodeoxynucleotide in von Frey test and hotplate test. Chromatin immunoprecipitation with quantitative PCR assay showed that CREB knockdown reduced the interaction of pCREB with the ccdc109a gene (encoding MCU expression) promoter and decreased the MCU mRNA transcription. RNA immunoprecipitation assay suggested that CPEB1 binds to the MCU mRNA 3' untranslated region. CPEB1 knockdown decreased the expression of MCU protein.
Conclusions: These findings suggest that spinal MCU is regulated by pCREB and CPEB1 in morphine tolerance, and that inhibition of MCU, pCREB, or CPEB1 may be useful in preventing the development of opioid tolerance.
Keywords: CPEB; CREB; calcium; mitochondrial calcium uniporter; morphine; opioid tolerance; opioids.
Copyright © 2019 British Journal of Anaesthesia. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures
Similar articles
-
Phospho-CREB Regulation on NMDA Glutamate Receptor 2B and Mitochondrial Calcium Uniporter in the Ventrolateral Periaqueductal Gray Controls Chronic Morphine Withdrawal in Male Rats.J Neurosci. 2025 Jul 16;45(29):e1934242025. doi: 10.1523/JNEUROSCI.1934-24.2025. J Neurosci. 2025. PMID: 40494620
-
Antisense oligonucleotide knockdown of mGlu₅ receptor attenuates the antinociceptive tolerance and up-regulated expression of spinal protein kinase C associated with chronic morphine treatment.Eur J Pharmacol. 2012 May 15;683(1-3):78-85. doi: 10.1016/j.ejphar.2012.02.046. Epub 2012 Mar 12. Eur J Pharmacol. 2012. PMID: 22429573
-
Phosphorylated CCAAT/Enhancer Binding Protein β Contributes to Rat HIV-Related Neuropathic Pain: In Vitro and In Vivo Studies.J Neurosci. 2018 Jan 17;38(3):555-574. doi: 10.1523/JNEUROSCI.3647-16.2017. Epub 2017 Dec 1. J Neurosci. 2018. PMID: 29196315 Free PMC article.
-
Contribution of adrenomedullin to the switch of G protein-coupled μ-opioid receptors from Gi to Gs in the spinal dorsal horn following chronic morphine exposure in rats.Br J Pharmacol. 2016 Apr;173(7):1196-207. doi: 10.1111/bph.13419. Epub 2016 Feb 25. Br J Pharmacol. 2016. PMID: 26750148 Free PMC article.
-
Activation of ERK/CREB pathway in spinal cord contributes to chronic constrictive injury-induced neuropathic pain in rats.Acta Pharmacol Sin. 2005 Jul;26(7):789-98. doi: 10.1111/j.1745-7254.2005.00123.x. Acta Pharmacol Sin. 2005. PMID: 15960884
Cited by
-
MCU Upregulation Overactivates Mitophagy by Promoting VDAC1 Dimerization and Ubiquitination in the Hepatotoxicity of Cadmium.Adv Sci (Weinh). 2023 Mar;10(7):e2203869. doi: 10.1002/advs.202203869. Epub 2023 Jan 15. Adv Sci (Weinh). 2023. PMID: 36642847 Free PMC article.
-
Mitochondrial calcium exchange in physiology and disease.Physiol Rev. 2022 Apr 1;102(2):893-992. doi: 10.1152/physrev.00041.2020. Epub 2021 Oct 26. Physiol Rev. 2022. PMID: 34698550 Free PMC article. Review.
-
Mitochondrial Calcium Uniporter (MCU)-Mediated Calcium Overload in Psychoactive Drug Neurotoxicity: From Pathogenesis to Therapeutic Targets.Int J Mol Sci. 2025 May 15;26(10):4732. doi: 10.3390/ijms26104732. Int J Mol Sci. 2025. PMID: 40429873 Free PMC article. Review.
-
Phospho-CREB Regulation on NMDA Glutamate Receptor 2B and Mitochondrial Calcium Uniporter in the Ventrolateral Periaqueductal Gray Controls Chronic Morphine Withdrawal in Male Rats.J Neurosci. 2025 Jul 16;45(29):e1934242025. doi: 10.1523/JNEUROSCI.1934-24.2025. J Neurosci. 2025. PMID: 40494620
-
Cataloguing and Selection of mRNAs Localized to Dendrites in Neurons and Regulated by RNA-Binding Proteins in RNA Granules.Biomolecules. 2020 Jan 22;10(2):167. doi: 10.3390/biom10020167. Biomolecules. 2020. PMID: 31978946 Free PMC article. Review.
References
-
- O’Brien P.L., Karnell L.H., Gokhale M., Kenneth Pack B.S., Campopiano M., Zur J. Prescribing of benzodiazepines and opioids to individuals with substance use disorders. Drug Alcohol Depend. 2017;178:223–230. - PubMed
-
- Volkow N.D., McLellan A.T. Opioid abuse in chronic pain—misconceptions and mitigation strategies. N Engl J Med. 2016;374:1253–1263. - PubMed
-
- Frenk S.M., Porter K.S., Paulozzi L.J. Prescription opioid analgesic use among adults: United States, 1999–2012. NCHS Data Brief. 2015;1–8 - PubMed
-
- Miller M., Barber C.W., Leatherman S. Prescription opioid duration of action and the risk of unintentional overdose among patients receiving opioid therapy. JAMA Intern Med. 2015;175:608–615. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

