Intracellular Zn2+ transients modulate global gene expression in dissociated rat hippocampal neurons
- PMID: 31253848
- PMCID: PMC6598991
- DOI: 10.1038/s41598-019-45844-2
Intracellular Zn2+ transients modulate global gene expression in dissociated rat hippocampal neurons
Abstract
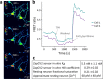
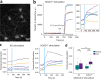
Zinc (Zn2+) is an integral component of many proteins and has been shown to act in a regulatory capacity in different mammalian systems, including as a neurotransmitter in neurons throughout the brain. While Zn2+ plays an important role in modulating neuronal potentiation and synaptic plasticity, little is known about the signaling mechanisms of this regulation. In dissociated rat hippocampal neuron cultures, we used fluorescent Zn2+ sensors to rigorously define resting Zn2+ levels and stimulation-dependent intracellular Zn2+ dynamics, and we performed RNA-Seq to characterize Zn2+-dependent transcriptional effects upon stimulation. We found that relatively small changes in cytosolic Zn2+ during stimulation altered expression levels of 931 genes, and these Zn2+ dynamics induced transcription of many genes implicated in neurite expansion and synaptic growth. Additionally, while we were unable to verify the presence of synaptic Zn2+ in these cultures, we did detect the synaptic vesicle Zn2+ transporter ZnT3 and found it to be substantially upregulated by cytosolic Zn2+ increases. These results provide the first global sequencing-based examination of Zn2+-dependent changes in transcription and identify genes that may mediate Zn2+-dependent processes and functions.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

