Perspectives from Young South African and Zimbabwean Women on Attributes of Four (Placebo) Vaginal Microbicide Delivery Forms
- PMID: 31254190
- PMCID: PMC6988116
- DOI: 10.1007/s10461-019-02576-8
Perspectives from Young South African and Zimbabwean Women on Attributes of Four (Placebo) Vaginal Microbicide Delivery Forms
Abstract
Introduction: Incorporating end-user input into the design of new vaginal microbicides for women is key to optimizing their uptake, consistent use, and, ultimately, success in combatting the heterosexual HIV epidemic.
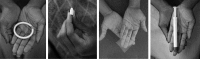
Methods: The Quatro Study assessed four placebo forms of vaginally inserted HIV-microbicides among young microbicide-naïve African women: on-demand film, insert and gel, and monthly ring. Participants randomly used each product for 1 month and provided product satisfaction ratings (1-5 scale), and opinions on product attributes and potential alternative designs. Qualitative data were collected through focus group discussions at study exit. Multivariable associations between attribute opinions and overall product rating were examined using Poisson regression models with robust standard errors to assess the attributes most influential to satisfaction.
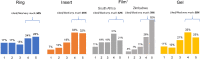
Results: Overall opinions of products and their individual attributes were generally positive; all products were rated either 4 or a 5 by ≥ 50% of participants. Attributes related to ease of use and interference with normal activities were the most salient predictors of satisfaction. Preferences for duration of use tended toward relatively shorter use periods for the ring (i.e., 1-3 months vs. 12 months) and for coitally independent dosing for the on-demand products.
Conclusions: How well a product fit in with participants' lifestyles was important to their overall satisfaction. For on-demand products, greater flexibility around timing of use was desired, to avoid coital dependency of the dosing.
Keywords: Acceptability; Africa; African women; End-user research; HIV prevention; Microbicides; Product attributes.
Figures
References
-
- Rees H, Delany-Morelwe S, Lombard C, et al. FACTS 001 Phase III trial of pericoital tenofovir 1% gel for HIV prevention in women. In: Paper presented at: Conference on Retroviruses and Opportunistic Infections, Boston 2015.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

