The prognostic factors between different viral etiologies among advanced hepatocellular carcinoma patients receiving sorafenib treatment
- PMID: 31254328
- PMCID: PMC11900675
- DOI: 10.1002/kjm2.12105
The prognostic factors between different viral etiologies among advanced hepatocellular carcinoma patients receiving sorafenib treatment
Abstract
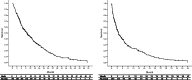
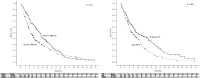
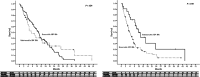
Sorafenib is currently the first-line therapy for advanced hepatocellular carcinoma (aHCC) patients. However, the outcomes and prognostic factors of sorafenib therapy have not been well investigated. We aimed to investigate the pretreatment factors and outcomes among Taiwanese aHCC patients receiving sorafenib treatment. A total of 347 patients with aHCC and well-compensated liver cirrhosis (Child-Pugh A) status receiving sorafenib were consecutively enrolled from March 2013 through December 2016. Pre-treatment clinical data and viral hepatitis markers were collected and analyzed with their outcomes. The primary endpoint of the study was overall survival. The factors associated with overall survival were also investigated. The median overall survival of all the patients was 238 days (range, 9-1504 days) with a 1-year overall survival of 43.2%. Positive hepatitis B surface antigen and absence of portal vein thrombosis (PVT) were independent factors associated with better overall survival. The median duration of sorafenib therapy was 93.0 days (range, 4-1504 days). After stopping sorafenib, the median survival was 93.0 days (range, 1-1254 days). The 1-year survival after stopping sorafenib was 21.2%. In chronic hepatitis B patients, total bilirubin level was the only factor associated with overall survival. Hepatitis C antibody RNA negativity, tumor size, PVT, and white blood cell count were the independent factors associated with survival among those chronic hepatitis C patients. There were different prognostic factors stratified by viral etiologies in aHCC patients receiving sorafenib. Viral eradication increased survival in chronic hepatitis C patients.
Keywords: advance; hepatocellular carcinoma; sorafenib; survival.
© 2019 The Authors. The Kaohsiung Journal of Medical Sciences published by John Wiley & Sons Australia on behalf of Kaohsiung Medical University.
Conflict of interest statement
All authors declare no potential conflict of interest.
Figures

Similar articles
-
Validation of the albumin-bilirubin grade-based integrated model as a predictor for sorafenib-failed hepatocellular carcinoma.Liver Int. 2018 Feb;38(2):321-330. doi: 10.1111/liv.13527. Epub 2017 Aug 9. Liver Int. 2018. PMID: 28736952
-
Lactate dehydrogenase is a prognostic indicator in patients with hepatocellular carcinoma treated by sorafenib: results from the real life practice in HBV endemic area.Oncotarget. 2016 Dec 27;7(52):86630-86647. doi: 10.18632/oncotarget.13428. Oncotarget. 2016. PMID: 27880930 Free PMC article.
-
Prognostic factors in patients with advanced hepatocellular carcinoma treated with sorafenib: a retrospective comparison with previously known prognostic models.Oncology. 2011;80(3-4):167-74. doi: 10.1159/000327591. Epub 2011 Jun 24. Oncology. 2011. PMID: 21701230
-
Sorafenib as first-line therapy in patients with advanced Child-Pugh B hepatocellular carcinoma-a meta-analysis.Eur J Cancer. 2018 Dec;105:1-9. doi: 10.1016/j.ejca.2018.09.031. Epub 2018 Oct 29. Eur J Cancer. 2018. PMID: 30384012
-
Outcomes of hepatocellular carcinoma patients treated with sorafenib: a meta-analysis of Phase III trials.Future Oncol. 2019 Oct;15(29):3411-3422. doi: 10.2217/fon-2019-0287. Epub 2019 Oct 7. Future Oncol. 2019. PMID: 31588789
Cited by
-
Potential Impact of IMbrave150 Results in the Evolving Treatment Landscape of Advanced Hepatocellular Carcinoma: A Multidisciplinary Expert Opinion.J Hepatocell Carcinoma. 2020 Dec 21;7:423-433. doi: 10.2147/JHC.S274930. eCollection 2020. J Hepatocell Carcinoma. 2020. PMID: 33376711 Free PMC article.
-
Eradication of hepatitis C virus preserve liver function and prolong survival in advanced hepatocellular carcinoma patients with limited life expectancy.Kaohsiung J Med Sci. 2021 Feb;37(2):145-153. doi: 10.1002/kjm2.12303. Epub 2020 Oct 6. Kaohsiung J Med Sci. 2021. PMID: 33022892 Free PMC article.
-
Sorafenib use in hepatitis B virus- or hepatitis C virus-related hepatocellular carcinoma: A propensity score matching study.Kaohsiung J Med Sci. 2021 Oct;37(10):894-902. doi: 10.1002/kjm2.12413. Epub 2021 Jun 24. Kaohsiung J Med Sci. 2021. PMID: 34166565 Free PMC article.
-
Significant down-regulation of growth hormone receptor expression revealed as a new unfavorable prognostic factor in hepatitis C virus-related hepatocellular carcinoma.Clin Mol Hepatol. 2021 Apr;27(2):313-328. doi: 10.3350/cmh.2020.0247. Epub 2020 Dec 14. Clin Mol Hepatol. 2021. PMID: 33317258 Free PMC article.
-
Unawareness of hepatitis B infection and lack of surveillance are associated with severity of hepatocellular carcinoma.Kaohsiung J Med Sci. 2023 Nov;39(11):1145-1154. doi: 10.1002/kjm2.12744. Epub 2023 Sep 2. Kaohsiung J Med Sci. 2023. PMID: 37658712 Free PMC article.
References
-
- Bosch FX, Ribes J, Diaz M, Cleries R. Primary liver cancer: Worldwide incidence and trends. Gastroenterology. 2004;127(5 Suppl 1):S5–S16. - PubMed
-
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. - PubMed
-
- Llovet JM, Schwartz M, Mazzaferro V. Resection and liver transplantation for hepatocellular carcinoma. Semin Liver Dis. 2005;25(2):181–200. - PubMed
-
- Shiina S, Teratani T, Obi S, Sato S, Tateishi R, Fujishima T, et al. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology. 2005;129(1):122–130. - PubMed
-
- Llovet JM, Real MI, Montana X, Planas R, Coll S, Aponte J, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: A randomised controlled trial. Lancet. 2002;359(9319):1734–1739. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

