Extension of the Pompe mutation database by linking disease-associated variants to clinical severity
- PMID: 31254424
- PMCID: PMC6851659
- DOI: 10.1002/humu.23854
Extension of the Pompe mutation database by linking disease-associated variants to clinical severity
Abstract
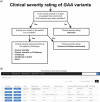
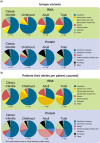
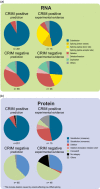
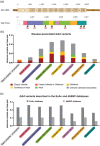
Pompe disease is an autosomal recessive lysosomal storage disorder caused by disease-associated variants in the acid alpha-glucosidase (GAA) gene. The current Pompe mutation database provides a severity rating of GAA variants based on in silico predictions and expression studies. Here, we extended the database with clinical information of reported phenotypes. We added additional in silico predictions for effects on splicing and protein function and for cross reactive immunologic material (CRIM) status, minor allele frequencies, and molecular analyses. We analyzed 867 patients and 562 GAA variants. Based on their combination with a GAA null allele (i.e., complete deficiency of GAA enzyme activity), 49% of the 422 disease-associated variants could be linked to classic infantile, childhood, or adult phenotypes. Predictions and immunoblot analyses identified 131 CRIM negative and 216 CRIM positive variants. While disease-associated missense variants were found throughout the GAA protein, they were enriched up to seven-fold in the catalytic site. Fifteen percent of disease-associated missense variants were predicted to affect splicing. This should be confirmed using splicing assays. Inclusion of clinical severity rating in the Pompe mutation database provides an invaluable tool for diagnosis, prognosis of disease progression, treatment regimens, and the future development of personalized medicine for Pompe disease.
Keywords: cardiac and skeletal muscle disorder; genotype-phenotype relationship; glycogen storage disease type II; lysosomal storage disease; www.pompecenter.nl.
© 2019 The Authors. Human Mutation Published by Wiley Periodicals, Inc.
Conflict of interest statement
AvdP has provided consulting services for various industries in the field of Pompe disease under an agreement between these industries and Erasmus MC, Rotterdam, The Netherlands.
Figures
References
-
- Bergsma, A. J. , van der Wal, E. , Broeders, M. , van der Ploeg, A. T. , & Pim Pijnappel, W. W. M. (2018). Alternative splicing in genetic diseases: Improved diagnosis and novel treatment options. International Review of Cell and Molecular Biology, 335, 85–141. https://doi.org/S1937-6448(17)30083-7 - PubMed
-
- Broomfield, A. , Fletcher, J. , Davison, J. , Finnegan, N. , Fenton, M. , Chikermane, A. , & Vellodi, A. (2016). Response of 33 UK patients with infantile‐onset Pompe disease to enzyme replacement therapy. Journal of Inherited Metabolic Disease (Dordrecht), 39(2), 261–271. 10.1007/s10545-015-9898-5. [pii] 10.1007/s10545‐015‐9898‐5. - DOI - PubMed
-
- van Capelle, C. I. , van der Beek, N. A. , Hagemans, M. L. , Arts, W. F. , Hop, W. C. , Lee, P. , & van der Ploeg, A. T. (2010). Effect of enzyme therapy in juvenile patients with Pompe disease: A three‐year open‐label study. Neuromuscular Disorders: NMD, 20(12), 775–782. https://doi.org/S0960-8966(10)00572-9 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

