Establishment of a novel experimental model for muscle-invasive bladder cancer using a dog bladder cancer organoid culture
- PMID: 31254429
- PMCID: PMC6726682
- DOI: 10.1111/cas.14118
Establishment of a novel experimental model for muscle-invasive bladder cancer using a dog bladder cancer organoid culture
Abstract
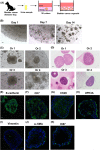
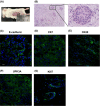
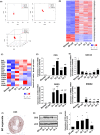
In human and dogs, bladder cancer (BC) is the most common neoplasm affecting the urinary tract. Dog BC resembles human muscle-invasive BC in histopathological characteristics and gene expression profiles, and could be an important research model for this disease. Cancer patient-derived organoid culture can recapitulate organ structures and maintains the gene expression profiles of original tumor tissues. In a previous study, we generated dog prostate cancer organoids using urine samples, however dog BC organoids had never been produced. Therefore we aimed to generate dog BC organoids using urine samples and check their histopathological characteristics, drug sensitivity, and gene expression profiles. Organoids from individual BC dogs were successfully generated, expressed urothelial cell markers (CK7, CK20, and UPK3A) and exhibited tumorigenesis in vivo. In a cell viability assay, the response to combined treatment with a range of anticancer drugs (cisplatin, vinblastine, gemcitabine or piroxicam) was markedly different in each BC organoid. In RNA-sequencing analysis, expression levels of basal cell markers (CK5 and DSG3) and several novel genes (MMP28, CTSE, CNN3, TFPI2, COL17A1, and AGPAT4) were upregulated in BC organoids compared with normal bladder tissues or two-dimensional (2D) BC cell lines. These established dog BC organoids might be a useful tool, not only to determine suitable chemotherapy for BC diseased dogs but also to identify novel biomarkers in human muscle-invasive BC. In the present study, for the 1st time, dog BC organoids were generated and several specifically upregulated organoid genes were identified. Our data suggest that dog BC organoids might become a new tool to provide fresh insights into both dog BC therapy and diagnostic biomarkers.
Keywords: RNA-seq; biomarker; bladder cancer; dog; organoid.
© 2019 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Figures



References
-
- Knapp DW, McMillan SK. Tumors of the urinary system In: MacEwen's W, ed. Small Animal Clinical Oncology, 5th ed St. Louis, MO: Elsevier–Saunders; 2013:572‐582.
-
- Lerner SP, Schoenberg MP, Sternberg CN. Textbook of Bladder Cancer. Oxon: Taylor and Francis; 2006.
-
- Patrick DJ, Fitzgerald SD, Sesterhenn IA, Davis CJ, Kiupel M. Classification of canine urinary bladder urothelial tumours based on the World Health Organization/International Society of Urological Pathology consensus classification. J Comp Pathol. 2006;135:190‐199. - PubMed
-
- Valli VE, Norris A, Jacobs RM, et al. Pathology of canine bladder and urethral cancer and correlation with tumour progression and survival. J Comp Pathol. 1995;113:113‐130. - PubMed
-
- Knapp D, McMillan S. Withrow and MacEwen's Small Animal Clinical Oncology. St Louis, MO: Elsevier‐Saunders; 2013.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

