From Perception Threshold to Ion Channels-A Computational Study
- PMID: 31255293
- PMCID: PMC6700668
- DOI: 10.1016/j.bpj.2019.04.041
From Perception Threshold to Ion Channels-A Computational Study
Abstract
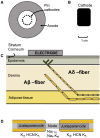
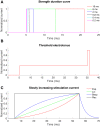
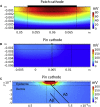
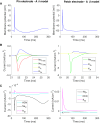
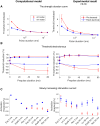
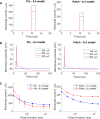
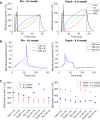
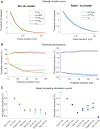
Small-surface-area electrodes have successfully been used to preferentially activate cutaneous nociceptors, unlike conventional large area-electrodes, which preferentially activate large non-nociceptor fibers. Assessments of the strength-duration relationship, threshold electrotonus, and slowly increasing pulse forms have displayed different perception thresholds between large and small surface electrodes, which may indicate different excitability properties of the activated cutaneous nerves. In this study, the origin of the differences in perception thresholds between the two electrodes was investigated. It was hypothesized that different perception thresholds could be explained by the varying distributions of voltage-gated ion channels and by morphological differences between peripheral nerve endings of small and large fibers. A two-part computational model was developed to study activation of peripheral nerve fibers by different cutaneous electrodes. The first part of the model was a finite-element model, which calculated the extracellular field delivered by the cutaneous electrodes. The second part of the model was a detailed multicompartment model of an Aδ-axon as well as an Aβ-axon. The axon models included a wide range of voltage-gated ion channels: NaTTXs, NaTTXr, Nap, Kdr, KM, KA, and HCN channel. The computational model reproduced the experimentally assessed perception thresholds for the three protocols, the strength-duration relationship, the threshold electrotonus, and the slowly increasing pulse forms. The results support the hypothesis that voltage-gated ion channel distributions and morphology differences between small and large fibers were sufficient to explain the difference in perception thresholds between the two electrodes. In conclusion, assessments of perception thresholds using the three protocols may be an indirect measurement of the membrane excitability, and computational models may have the possibility to link voltage-gated ion channel activation to perception threshold measurements.
Copyright © 2019 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Serra J., Bostock H., Quiles C. Microneurographic identification of spontaneous activity in C-nociceptors in neuropathic pain states in humans and rats. Pain. 2012;153:42–55. - PubMed
-
- Portenoy R.K., Ugarte C., Haas G. Population-based survey of pain in the United States: differences among white, African American, and Hispanic subjects. J. Pain. 2004;5:317–328. - PubMed
-
- Waxman S.G., Zamponi G.W. Regulating excitability of peripheral afferents: emerging ion channel targets. Nat. Neurosci. 2014;17:153–163. - PubMed
-
- Gold M.S., Shuster M.J., Levine J.D. Characterization of six voltage-gated K+ currents in adult rat sensory neurons. J. Neurophysiol. 1996;75:2629–2646. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

