Transneuronal Propagation of Pathologic α-Synuclein from the Gut to the Brain Models Parkinson's Disease
- PMID: 31255487
- PMCID: PMC6706297
- DOI: 10.1016/j.neuron.2019.05.035
Transneuronal Propagation of Pathologic α-Synuclein from the Gut to the Brain Models Parkinson's Disease
Abstract
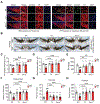
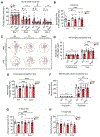
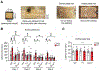
Analysis of human pathology led Braak to postulate that α-synuclein (α-syn) pathology could spread from the gut to brain via the vagus nerve. Here, we test this postulate by assessing α-synucleinopathy in the brain in a novel gut-to-brain α-syn transmission mouse model, where pathological α-syn preformed fibrils were injected into the duodenal and pyloric muscularis layer. Spread of pathologic α-syn in brain, as assessed by phosphorylation of serine 129 of α-syn, was observed first in the dorsal motor nucleus, then in caudal portions of the hindbrain, including the locus coeruleus, and much later in basolateral amygdala, dorsal raphe nucleus, and the substantia nigra pars compacta. Moreover, loss of dopaminergic neurons and motor and non-motor symptoms were observed in a similar temporal manner. Truncal vagotomy and α-syn deficiency prevented the gut-to-brain spread of α-synucleinopathy and associated neurodegeneration and behavioral deficits. This study supports the Braak hypothesis in the etiology of idiopathic Parkinson's disease (PD).
Keywords: Braak hypothesis; Lewy body pathology; Parkinson’s disease; gut to brain transmission; motor symptoms; neurodegeneration; non-motor symptoms; pre-formed fibrils; vagus nerve; α-synuclein.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
DECLARATION OF INTERESTS
The authors declare no competing interests.
Figures



Comment in
-
New models show gut-brain transmission of Parkinson disease pathology.Nat Rev Neurol. 2019 Sep;15(9):491. doi: 10.1038/s41582-019-0241-x. Nat Rev Neurol. 2019. PMID: 31312039 No abstract available.
-
Exploring the Peripheral Initiation of Parkinson's Disease in Animal Models.Neuron. 2019 Aug 21;103(4):547-549. doi: 10.1016/j.neuron.2019.07.031. Neuron. 2019. PMID: 31437447
-
Lots of Movement in Gut and Parkinson's Research.Trends Endocrinol Metab. 2019 Oct;30(10):687-689. doi: 10.1016/j.tem.2019.08.001. Epub 2019 Aug 28. Trends Endocrinol Metab. 2019. PMID: 31473011
References
-
- Anderson JP, Walker DE, Goldstein JM, de Laat R, Banducci K, Caccavello RJ, Barbour R, Huang J, Kling K, Lee M, et al. (2006). Phosphorylation of Ser-129 is the dominant pathological modification of alpha-synuclein in familial and sporadic Lewy body disease. J Biol Chem 281, 29739–29752. - PubMed
-
- Arias N, Mendez M, and Arias JL (2015). The importance of the context in the hippocampus and brain related areas throughout the performance of a fear conditioning task. Hippocampus 25, 1242–1249. - PubMed
-
- Berthoud HR, Carlson NR, and Powley TL (1991). Topography of efferent vagal innervation of the rat gastrointestinal tract. Am J Physiol 260, R200–207. - PubMed
-
- Bevins RA, and Besheer J (2006). Object recognition in rats and mice: a one-trial-non-matching-to-sample learning task to study ‘recognition memory’. Nat Protoc 1, 1306–1311. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

