Rational approaches, design strategies, structure activity relationship and mechanistic insights for therapeutic coumarin hybrids
- PMID: 31255497
- PMCID: PMC7970831
- DOI: 10.1016/j.bmc.2019.06.033
Rational approaches, design strategies, structure activity relationship and mechanistic insights for therapeutic coumarin hybrids
Abstract
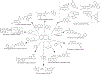
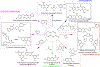
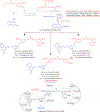
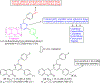
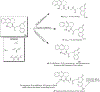
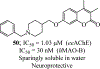
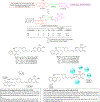
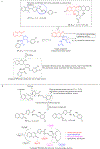
Hybrid molecules, furnished by combining two or more pharmacophores is an emerging concept in the field of medicinal chemistry and drug discovery that has attracted substantial traction in the past few years. Naturally occurring scaffolds such as coumarins display a wide spectrum of pharmacological activities including anticancer, antibiotic, antidiabetic and others, by acting on multiple targets. In this view, various coumarin-based hybrids possessing diverse medicinal attributes were synthesized in the last five years by conjugating coumarin moiety with other therapeutic pharmacophores. The current review summarizes the recent development (2014 and onwards) of these pharmacologically active coumarin hybrids and demonstrates rationale behind their design, structure-activity relationships (SAR) and mechanistic studies performed on these hybrid molecules. This review will be beneficial for medicinal chemist and chemical biologist, and in general to the drug discovery community and will facilitate the synthesis and development of novel, potent coumarin hybrid molecules serving as lead molecules for the treatment of complex disorders.
Keywords: Anti-Alzheimer’s; Anti-inflammatory; Anticancer; Antidiabetic; Antimicrobial; Antioxidant; Coumarin hybrids; Design strategies; Mechanistic insights; Structure-activity relationship.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflict of interest
The authors declare no conflict of interest.
Figures


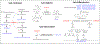
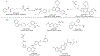


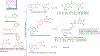


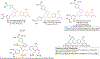


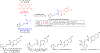

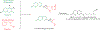


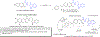



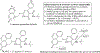




References
-
- Kaur P, Arora R, Gill NS, Review on oxygen heterocycles, Indo Am. J. Pharma. Res. 3 (2013) 9067–9084. 10.1044/1980-iajpr.01110 - DOI
-
- Feuer G, The metabolism and biological actions of coumarins. In Progress in Medicinal Chemistry (Elis GP, and West GB, Ed.), North Holland, New York. 1974, Vol 10, pp 86–87 - PubMed
-
- Evans WC, Trease and Evans’ Pharmacognosy, Elsevier Health Sciences, 2009.
-
- Rosselli S, Maggio AM, Faraone N, Spadaro V, Morris-Natschke SL, Bastow KF, Lee KH, Bruno M, The cytotoxic properties of natural coumarins isolated from roots of Ferulago campestris (Apiaceae) and of synthetic ester derivatives of aegelinol, Nat. Prod. Commun. 4 (2009) 1701–1706. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Chemical Information
Miscellaneous

