Oral insulin therapy for primary prevention of type 1 diabetes in infants with high genetic risk: the GPPAD-POInT (global platform for the prevention of autoimmune diabetes primary oral insulin trial) study protocol
- PMID: 31256036
- PMCID: PMC6609035
- DOI: 10.1136/bmjopen-2018-028578
Oral insulin therapy for primary prevention of type 1 diabetes in infants with high genetic risk: the GPPAD-POInT (global platform for the prevention of autoimmune diabetes primary oral insulin trial) study protocol
Abstract
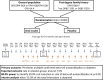
Introduction: The POInT study, an investigator initiated, randomised, placebo-controlled, double-blind, multicentre primary prevention trial is conducted to determine whether daily administration of oral insulin, from age 4.0 months to 7.0 months until age 36.0 months to children with elevated genetic risk for type 1 diabetes, reduces the incidence of beta-cell autoantibodies and diabetes.
Methods and analysis: Infants aged 4.0 to 7.0 months from Germany, Poland, Belgium, UK and Sweden are eligible if they have a >10.0% expected risk for developing multiple beta-cell autoantibodies as determined by genetic risk score or family history and human leucocyte antigen genotype. Infants are randomised 1:1 to daily oral insulin (7.5 mg for 2 months, 22.5 mg for 2 months, 67.5 mg until age 36.0 months) or placebo, and followed for a maximum of 7 years. Treatment and follow-up is stopped if a child develops diabetes. The primary outcome is the development of persistent confirmed multiple beta-cell autoantibodies or diabetes. Other outcomes are: (1) Any persistent confirmed beta-cell autoantibody (glutamic acid decarboxylase (GADA), IA-2A, autoantibodies to insulin (IAA) and zinc transporter 8 or tetraspanin 7), or diabetes, (2) Persistent confirmed IAA, (3) Persistent confirmed GADA and (4) Abnormal glucose tolerance or diabetes.
Ethics and dissemination: The study is approved by the ethical committees of all participating clinical sites. The results will be disseminated through peer-reviewed journals and conference presentations and will be openly shared after completion of the trial.
Trial registration number: NCT03364868.
Keywords: clinical trials; general diabetes; paediatric endocrinology.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: Matthew Snape has received research grants paid to his institution for work as an investigator on clinical trials from the GSK group of companies, Pfizer, Janssen, Novavax, MedImmune, Alios BioPharma and Ablynx. He has also received support for travel and accommodation to attend international conferences from GSK group of companies, and, prior to 2017, received support paid to his institution as a member of the advisory board for Sanofi-Pasteur MSD and as a consultant for MedImmune.
Figures
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical