β-catenin-mediated hair growth induction effect of 3,4,5-tri- O-caffeoylquinic acid
- PMID: 31256073
- PMCID: PMC6628991
- DOI: 10.18632/aging.102048
β-catenin-mediated hair growth induction effect of 3,4,5-tri- O-caffeoylquinic acid
Abstract
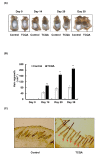
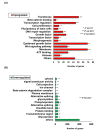
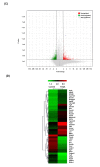
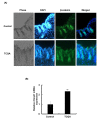
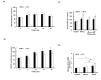
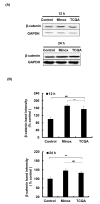
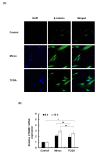
The hair follicle is a complex structure that goes through a cyclic period of growth (anagen), regression (catagen), and rest (telogen) under the regulation of several signaling pathways, including Wnt/ β-catenin, FGF, Shh, and Notch. The Wnt/β-catenin signaling is specifically involved in hair follicle morphogenesis, regeneration, and growth. β-catenin is expressed in the dermal papilla and promotes anagen induction and duration, as well as keratinocyte regulation and differentiation. In this study, we demonstrated the activation of β-catenin by a polyphenolic compound 3,4,5-tri-O-caffeoylquinic acid (TCQA) in mice model and in human dermal papilla cells to promote hair growth cycle. A complete regrowth of the shaved area of C3H mice was observed upon treatment with TCQA. Global gene expression analysis using microarray showed an upregulation in hair growth-associated genes. Moreover, the expression of β-catenin was remarkably upregulated in vivo and in vitro. These findings suggest that β-catenin activation by TCQA promoted the initiation of the anagen phase of the hair cycle.
Keywords: 3,4,5-tri--caffeoylquinic acid (TCQA) O; Wnt/ β-catenin pathway; anagen; dermal papilla; β-catenin.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

