Phenobarbital, Midazolam Pharmacokinetics, Effectiveness, and Drug-Drug Interaction in Asphyxiated Neonates Undergoing Therapeutic Hypothermia
- PMID: 31256150
- PMCID: PMC6878731
- DOI: 10.1159/000499330
Phenobarbital, Midazolam Pharmacokinetics, Effectiveness, and Drug-Drug Interaction in Asphyxiated Neonates Undergoing Therapeutic Hypothermia
Abstract
Background: Phenobarbital and midazolam are commonly used drugs in (near-)term neonates treated with therapeutic hypothermia for hypoxic-ischaemic encephalopathy, for sedation, and/or as anti-epileptic drug. Phenobarbital is an inducer of cytochrome P450 (CYP) 3A, while midazolam is a CYP3A substrate. Therefore, co-treatment with phenobarbital might impact midazolam clearance.
Objectives: To assess pharmacokinetics and clinical anti-epileptic effectiveness of phenobarbital and midazolam in asphyxiated neonates and to develop dosing guidelines.
Methods: Data were collected in the prospective multicentre PharmaCool study. In the present study, neonates treated with therapeutic hypothermia and receiving midazolam and/or phenobarbital were included. Plasma concentrations of phenobarbital and midazolam including its metabolites were determined in blood samples drawn on days 2-5 after birth. Pharmacokinetic analyses were performed using non-linear mixed effects modelling; clinical effectiveness was defined as no use of additional anti-epileptic drugs.
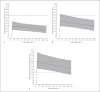
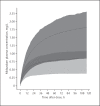
Results: Data were available from 113 (phenobarbital) and 118 (midazolam) neonates; 68 were treated with both medications. Only clearance of 1-hydroxy midazolam was influenced by hypothermia. Phenobarbital co-administration increased midazolam clearance by a factor 2.3 (95% CI 1.9-2.9, p < 0.05). Anticonvulsant effectiveness was 65.5% for phenobarbital and 37.1% for add-on midazolam.
Conclusions: Therapeutic hypothermia does not influence clearance of phenobarbital or midazolam in (near-)term neonates with hypoxic-ischaemic encephalopathy. A phenobarbital dose of 30 mg/kg is advised to reach therapeutic concentrations. Phenobarbital co-administration significantly increased midazolam clearance. Should phenobarbital be substituted by non-CYP3A inducers as first-line anticonvulsant, a 50% lower midazolam maintenance dose might be appropriate to avoid excessive exposure during the first days after birth.
Keywords: Hypoxic-ischaemic encephalopathy; Midazolam; Neonates; Pharmacokinetics; Phenobarbital.
© 2019 The Author(s) Published by S. Karger AG, Basel.
Figures


References
-
- Azzopardi DV, Strohm B, Edwards AD, Dyet L, Halliday HL, Juszczak E, et al. TOBY Study Group Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med. 2009 Oct;361((14)):1349–58. - PubMed
-
- Groenendaal F, Casaer A, Dijkman KP, Gavilanes AW, de Haan TR, ter Horst HJ, et al. Introduction of hypothermia for neonates with perinatal asphyxia in the Netherlands and Flanders. Neonatology. 2013;104((1)):15–21. - PubMed
-
- El-Dib M, Soul JS. The use of phenobarbital and other anti-seizure drugs in newborns. Semin Fetal Neonatal Med. 2017 Oct;22((5)):321–7. - PubMed
-
- van den Broek MP, Groenendaal F, Toet MC, van Straaten HL, van Hasselt JG, Huitema AD, et al. Pharmacokinetics and clinical efficacy of phenobarbital in asphyxiated newborns treated with hypothermia: a thermopharmacological approach. Clin Pharmacokinet. 2012 Oct;51((10)):671–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

