Regulation of Energy Expenditure by Brainstem GABA Neurons
- PMID: 31257028
- PMCID: PMC7481042
- DOI: 10.1016/j.cell.2019.05.048
Regulation of Energy Expenditure by Brainstem GABA Neurons
Abstract
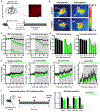
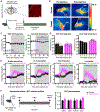
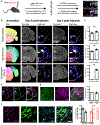
Homeostatic control of core body temperature is essential for survival. Temperature is sensed by specific neurons, in turn eliciting both behavioral (i.e., locomotion) and physiologic (i.e., thermogenesis, vasodilatation) responses. Here, we report that a population of GABAergic (Vgat-expressing) neurons in the dorsolateral portion of the dorsal raphe nucleus (DRN), hereafter DRNVgat neurons, are activated by ambient heat and bidirectionally regulate energy expenditure through changes in both thermogenesis and locomotion. We find that DRNVgat neurons innervate brown fat via a descending projection to the raphe pallidus (RPa). These neurons also densely innervate ascending targets implicated in the central regulation of energy expenditure, including the hypothalamus and extended amygdala. Optogenetic stimulation of different projection targets reveals that DRNVgat neurons are capable of regulating thermogenesis through both a "direct" descending pathway through the RPa and multiple "indirect" ascending pathways. This work establishes a key regulatory role for DRNVgat neurons in controlling energy expenditure.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
DECLARATION OF INTERESTS
The authors declare no competing interests.
Figures




Comment in
-
Keeping your cool.Nat Rev Neurosci. 2019 Sep;20(9):512-513. doi: 10.1038/s41583-019-0205-6. Nat Rev Neurosci. 2019. PMID: 31312034 No abstract available.
-
Cool(ing) brain stem GABA neurons.Cell Res. 2019 Oct;29(10):785-786. doi: 10.1038/s41422-019-0223-y. Cell Res. 2019. PMID: 31471559 Free PMC article. No abstract available.
References
-
- Cannon B, and Nedergaard J (2004). Brown adipose tissue: function and physiological significance. Physiol. Rev 84, 277–359. - PubMed
-
- Dib B, Rompre PP, Amir S, and Shizgal P (1994). Thermogenesis in brown adipose tissue is activated by electrical stimulation of the rat dorsal raphe nucleus. Brain Res. 650, 149–152. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

