Molecular basis of reduced LAIR1 expression in childhood severe malarial anaemia: Implications for leukocyte inhibitory signalling
- PMID: 31257148
- PMCID: PMC6642411
- DOI: 10.1016/j.ebiom.2019.06.040
Molecular basis of reduced LAIR1 expression in childhood severe malarial anaemia: Implications for leukocyte inhibitory signalling
Abstract
Background: Leukocyte-associated immunoglobulin like receptor-1 (LAIR1) is a transmembrane inhibitory receptor that influences susceptibility to a myriad of inflammatory diseases. Our recent investigations of severe malarial anaemia (SMA) pathogenesis in Kenyan children discovered that novel LAIR1 genetic variants which were associated with decreased LAIR1 transcripts enhanced the longitudinal risk of SMA and all-cause mortality.
Methods: To characterize the molecular mechanism(s) responsible for altered LAIR1 signalling in severe malaria, we determined LAIR1 transcripts and protein, sLAIR1, sLAIR2, and complement component 1q (C1q) in children with malarial anaemia, followed by a series of in vitro experiments investigating the LAIR1 signalling cascade.
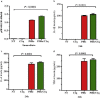
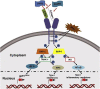
Findings: Kenyan children with SMA had elevated circulating levels of soluble LAIR1 (sLAIR1) relative to non-SMA (1.69-fold P < .0001). The LAIR1 antagonist, sLAIR2, was also elevated in the circulation of children with SMA (1.59 fold-change, P < .0001). There was a positive correlation between sLAIR1 and sLAIR2 (ρ = 0.741, P < .0001). Conversely, circulating levels of complement component 1q (C1q), a LAIR1 natural ligand, were lower in SMA (-1.21-fold P = .048). These in vivo findings suggest that reduced membrane-bound LAIR1 expression in SMA is associated with elevated production of sLAIR1, sLAIR2 (antagonist), and limited C1q (agonist) availability. Since reduced LAIR1 transcripts in SMA were associated with increased acquisition of haemozoin (PfHz) by monocytes (P = .028), we explored the relationship between acquisition of intraleukocytic PfHz, LAIR1 expression, and subsequent impacts on leukocyte signalling in cultured PBMCs from malaria-naïve donors stimulated with physiological concentrations of PfHz (10 μg/mL). Phagocytosis of PfHz reduced LAIR1 transcript and protein expression in a time-dependent manner (P < .050), and inhibited LAIR1 signalling through decreased phosphorylation of LAIR1 (P < .0001) and SH2-domain containing phosphatase-1 (SHP-1) (P < .001). This process was associated with NF-κB activation (P < .0001) and enhanced production of IL-6, IL-1β, and TNF-α (all P < .0001).
Interpretation: Collectively, these findings demonstrate that SMA is characterized by reduced LAIR1 transmembrane expression, reduced C1q, and enhanced production of sLAIR1 and sLAIR2, molecular events which can promote enhanced production of cytokines that contribute to the pathogenesis of SMA. These investigations are important for discovering immune checkpoints that could be future targets of immunotherapy to improve disease outcomes.
Keywords: Complement component 1q; Leukocyte-associated immunoglobulin like receptor-1; Leukocyte-associated immunoglobulin like receptor-2; Plasmodium falciparum haemozoin; Plasmodium falciparum malaria; Severe malarial anaemia.
Copyright © 2019. Published by Elsevier B.V.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Integrated OMICS platforms identify LAIR1 genetic variants as novel predictors of cross-sectional and longitudinal susceptibility to severe malaria and all-cause mortality in Kenyan children.EBioMedicine. 2019 Jul;45:290-302. doi: 10.1016/j.ebiom.2019.06.043. Epub 2019 Jul 2. EBioMedicine. 2019. PMID: 31278068 Free PMC article.
-
Acquisition of hemozoin by monocytes down-regulates interleukin-12 p40 (IL-12p40) transcripts and circulating IL-12p70 through an IL-10-dependent mechanism: in vivo and in vitro findings in severe malarial anemia.Infect Immun. 2006 Sep;74(9):5249-60. doi: 10.1128/IAI.00843-06. Infect Immun. 2006. PMID: 16926419 Free PMC article.
-
Role of monocyte-acquired hemozoin in suppression of macrophage migration inhibitory factor in children with severe malarial anemia.Infect Immun. 2007 Jan;75(1):201-10. doi: 10.1128/IAI.01327-06. Epub 2006 Oct 23. Infect Immun. 2007. PMID: 17060471 Free PMC article.
-
Severe malarial anemia: innate immunity and pathogenesis.Int J Biol Sci. 2011;7(9):1427-42. doi: 10.7150/ijbs.7.1427. Epub 2011 Nov 2. Int J Biol Sci. 2011. PMID: 22110393 Free PMC article. Review.
-
Anaemia of Plasmodium falciparum malaria.Baillieres Clin Haematol. 1992 Apr;5(2):315-30. doi: 10.1016/s0950-3536(11)80022-3. Baillieres Clin Haematol. 1992. PMID: 1511178 Review.
Cited by
-
Immunoregulatory complement receptor-1 and leukocyte-associated Ig-like receptor-1 expression on leukocytes in Psoriasis vulgaris.Innate Immun. 2020 Nov;26(8):683-692. doi: 10.1177/1753425920942570. Epub 2020 Jul 30. Innate Immun. 2020. PMID: 32731787 Free PMC article.
-
The Molecular Interaction of Collagen with Cell Receptors for Biological Function.Polymers (Basel). 2022 Feb 23;14(5):876. doi: 10.3390/polym14050876. Polymers (Basel). 2022. PMID: 35267698 Free PMC article. Review.
-
Ingestion of hemozoin by peripheral blood mononuclear cells alters temporal gene expression of ubiquitination processes.Biochem Biophys Rep. 2022 Jan 11;29:101207. doi: 10.1016/j.bbrep.2022.101207. eCollection 2022 Mar. Biochem Biophys Rep. 2022. PMID: 35071802 Free PMC article.
-
LAIR1 prevents excess inflammatory tissue damage in S. aureus skin infection and Cutaneous T-cell Lymphoma.bioRxiv [Preprint]. 2024 Jun 16:2024.06.13.598864. doi: 10.1101/2024.06.13.598864. bioRxiv. 2024. PMID: 38915487 Free PMC article. Preprint.
-
Differential Gene Expression in Host Ubiquitination Processes in Childhood Malarial Anemia.Front Genet. 2021 Nov 22;12:764759. doi: 10.3389/fgene.2021.764759. eCollection 2021. Front Genet. 2021. PMID: 34880904 Free PMC article.
References
-
- UNICEF. Malaria in Africa. 2018.
-
- WHO. World Malaria (Report. http://appswhoint/iris/bitstream/handle/10665/259492/9789241565523-engpdf;jsessionid=7C4AC215FEF706839BD95175ADE65ABA?sequence=1) 2017.
-
- Obonyo C.O., Vulule J., Akhwale W.S., Grobbee D.E. In-hospital morbidity and mortality due to severe malarial anemia in western Kenya. Am J Trop Med Hyg. 2007;77(6 Suppl):23–28. - PubMed
-
- Ong'echa J.M., Keller C.C., Were T., Ouma C., Otieno R.O., Landis-Lewis Z. Parasitemia, anemia, and malarial anemia in infants and young children in a rural holoendemic Plasmodium falciparum transmission area. Am J Trop Med Hyg. 2006;74(3):376–385. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

