EGFR-Pak Signaling Selectively Regulates Glutamine Deprivation-Induced Macropinocytosis
- PMID: 31257175
- PMCID: PMC6684838
- DOI: 10.1016/j.devcel.2019.05.043
EGFR-Pak Signaling Selectively Regulates Glutamine Deprivation-Induced Macropinocytosis
Abstract
Macropinocytosis has emerged as an important nutrient-scavenging pathway that supports tumor cell fitness. By internalizing extracellular protein and targeting it for lysosomal degradation, this endocytic pathway functions as an amino acid supply route, permitting tumor cell growth and survival despite the nutrient-poor conditions of the tumor microenvironment. Here, we provide evidence that a subset of pancreatic ductal adenocarcinoma (PDAC) tumors are wired to integrate contextual metabolic inputs to regulate macropinocytosis, dialing up or down this uptake pathway depending on nutrient availability. We find that regional depletion of amino acids coincides with increased levels of macropinocytosis and that the scarcity of glutamine uniquely drives this process. Mechanistically, this stimulation of macropinocytosis depends on the nutrient stress-induced potentiation of epidermal growth factor receptor signaling that, through the activation of Pak, controls the extent of macropinocytosis in these cells. These results provide a mechanistic understanding of how nutritional cues can control protein scavenging in PDAC tumors.
Keywords: EGFR; Pak; Ras; cancer metabolism; macropinocytosis; pancreatic cancer; protein scavenging.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
DECLARATION OF INTERESTS
C.C. is an inventor on a published patent application entitled “Cancer diagnostics, therapeutics, and drug discovery associated with macropinocytosis,” Pub. No.: US 2018/0335420 A1.
Figures
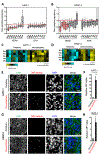
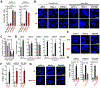
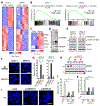
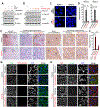
Comment in
-
EGFR Pathway Links Amino Acid Levels and Induction of Macropinocytosis.Dev Cell. 2019 Aug 5;50(3):261-263. doi: 10.1016/j.devcel.2019.07.020. Dev Cell. 2019. PMID: 31386860
References
-
- Brunk U, Schellens J, and Westermark B (1976). Influence of epidermal growth factor (EGF) on ruffling activity, pinocytosis and proliferation of cultivated human glia cells. Exp Cell Res 103, 295–302. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

