Cholinesterase inhibitors as Alzheimer's therapeutics (Review)
- PMID: 31257471
- PMCID: PMC6625431
- DOI: 10.3892/mmr.2019.10374
Cholinesterase inhibitors as Alzheimer's therapeutics (Review)
Abstract
Alzheimer's disease (AD) is one of the most common forms of dementia. AD is a chronic syndrome of the central nervous system that causes a decline in cognitive function and language ability. Cholinergic deficiency is associated with AD, and various cholinesterase inhibitors have been developed for the treatment of AD, including naturally‑derived inhibitors, synthetic analogues and hybrids. Currently, the available drugs for AD are predominantly cholinesterase inhibitors. However, the efficacy of these drugs is limited as they may cause adverse side effects and are not able to completely arrest the progression of the disease. Since AD is multifactorial disease, dual and multi‑target inhibitors have been developed. The clinical applications and the limitations of the inhibitors used to treat AD are discussed in the present review. Additionally, this review presents the current status and future directions for the development of novel drugs with reduced toxicity and preserved pharmacological activity.
Figures
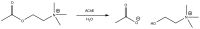
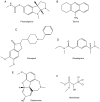
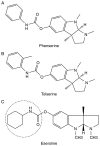
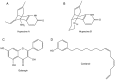
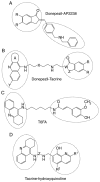
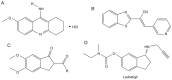
References
-
- Kuhn D. New horizons. Contemporary longterm care. 2003;26:25–26. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

