The Zebrafish Cytochrome b5/Cytochrome b5 Reductase/NADH System Efficiently Reduces Cytoglobins 1 and 2: Conserved Activity of Cytochrome b5/Cytochrome b5 Reductases during Vertebrate Evolution
- PMID: 31257865
- PMCID: PMC6657698
- DOI: 10.1021/acs.biochem.9b00406
The Zebrafish Cytochrome b5/Cytochrome b5 Reductase/NADH System Efficiently Reduces Cytoglobins 1 and 2: Conserved Activity of Cytochrome b5/Cytochrome b5 Reductases during Vertebrate Evolution
Abstract
Cytoglobin is a heme protein evolutionarily related to hemoglobin and myoglobin. Cytoglobin is expressed ubiquitously in mammalian tissues; however, its physiological functions are yet unclear. Phylogenetic analyses indicate that the cytoglobin gene is highly conserved in vertebrate clades, from fish to reptiles, amphibians, birds, and mammals. Most proposed roles for cytoglobin require the maintenance of a pool of reduced cytoglobin (FeII). We have shown previously that the human cytochrome b5/cytochrome b5 reductase system, considered a quintessential hemoglobin/myoglobin reductant, can reduce human and zebrafish cytoglobins ≤250-fold faster than human hemoglobin or myoglobin. It was unclear whether this reduction of zebrafish cytoglobins by mammalian proteins indicates a conserved pathway through vertebrate evolution. Here, we report the reduction of zebrafish cytoglobins 1 and 2 by the zebrafish cytochrome b5 reductase and the two zebrafish cytochrome b5 isoforms. In addition, the reducing system also supports reduction of Globin X, a conserved globin in fish and amphibians. Indeed, the zebrafish reducing system can maintain a fully reduced pool for both cytoglobins, and both cytochrome b5 isoforms can support this process. We determined the P50 for oxygen to be 0.5 Torr for cytoglobin 1 and 4.4 Torr for cytoglobin 2 at 25 °C. Thus, even at low oxygen tensions, the reduced cytoglobins may exist in a predominant oxygen-bound form. Under these conditions, the cytochrome b5/cytochrome b5 reductase system can support a conserved role for cytoglobins through evolution, providing electrons for redox signaling reactions such as nitric oxide dioxygenation, nitrite reduction, and phospholipid oxidation.
Conflict of interest statement
CONFLICT OF INTEREST STATEMENT
A.W.D., J.J.R., M.T.G. and J.T. are coinventors of provisional and pending patents for the use of recombinant cytoglobin, neuroglobin and other heme-based molecules as antidotes for carbon monoxide poisoning. Globin Solutions, Inc. has licensed this technology. J.J.R., M.T.G. and J.T. are shareholders and officers in Globin Solutions, Inc. J.J.R. is an officer and director of Globin Solutions, Inc. M.T.G. is a director and advisor of Globin Solutions, Inc. J.T. is an officer of Globin Solutions, Inc. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Figures

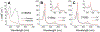

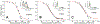
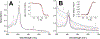

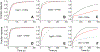
References
-
- Burmester T, and Hankeln T (2014) Function and evolution of vertebrate globins, Acta Physiol (Oxf) 211, 501–514. - PubMed
-
- Reeder BJ, Svistunenko DA, and Wilson MT (2011) Lipid binding to cytoglobin leads to a change in haem co-ordination: a role for cytoglobin in lipid signalling of oxidative stress, Biochem J 434, 483–492. - PubMed
-
- Tejero J, Kapralov AA, Baumgartner MP, Sparacino-Watkins CE, Anthonymuthu TS, Vlasova II, Camacho CJ, Gladwin MT, Bayir H, and Kagan VE (2016) Peroxidase Activation of Cytoglobin by Anionic phospholipids: Mechanisms and Consequences, Biochimica et Biophysica Acta-Molecular and Cell Biology of Lipids 1861, 391–401. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

