Removal of a Conserved Disulfide Bond Does Not Compromise Mechanical Stability of a VHH Antibody Complex
- PMID: 31257893
- PMCID: PMC6975629
- DOI: 10.1021/acs.nanolett.9b02062
Removal of a Conserved Disulfide Bond Does Not Compromise Mechanical Stability of a VHH Antibody Complex
Abstract
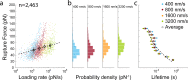
Single-domain VHH antibodies are promising reagents for medical therapy. A conserved disulfide bond within the VHH framework region is known to be critical for thermal stability, however, no prior studies have investigated its influence on the stability of VHH antibody-antigen complexes under mechanical load. Here, we used single-molecule force spectroscopy to test the influence of a VHH domain's conserved disulfide bond on the mechanical strength of the interaction with its antigen mCherry. We found that although removal of the disulfide bond through cysteine-to-alanine mutagenesis significantly lowered VHH domain denaturation temperature, it had no significant impact on the mechanical strength of the VHH:mCherry interaction with complex rupture occurring at ∼60 pN at 103-104 pN/sec regardless of disulfide bond state. These results demonstrate that mechanostable binding interactions can be built on molecular scaffolds that may be thermodynamically compromised at equilibrium.
Keywords: Antibodies; biophysics; disulfide bond; nanobody; single-molecule force spectroscopy.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Role of a noncanonical disulfide bond in the stability, affinity, and flexibility of a VHH specific for the Listeria virulence factor InlB.Protein Sci. 2020 Apr;29(4):1004-1017. doi: 10.1002/pro.3831. Epub 2020 Feb 8. Protein Sci. 2020. PMID: 31981247 Free PMC article.
-
The role of intra-domain disulfide bonds in heat-induced irreversible denaturation of camelid single domain VHH antibodies.J Biochem. 2016 Jan;159(1):111-21. doi: 10.1093/jb/mvv082. Epub 2015 Aug 19. J Biochem. 2016. PMID: 26289739 Free PMC article.
-
Dual beneficial effect of interloop disulfide bond for single domain antibody fragments.J Biol Chem. 2012 Jan 13;287(3):1970-9. doi: 10.1074/jbc.M111.242818. Epub 2011 Nov 29. J Biol Chem. 2012. PMID: 22128183 Free PMC article.
-
VHH antibodies: emerging reagents for the analysis of environmental chemicals.Anal Bioanal Chem. 2016 Sep;408(22):5985-6002. doi: 10.1007/s00216-016-9585-x. Epub 2016 May 21. Anal Bioanal Chem. 2016. PMID: 27209591 Free PMC article. Review.
-
Disulfide bonding in protein biophysics.Annu Rev Biophys. 2012;41:63-79. doi: 10.1146/annurev-biophys-050511-102321. Epub 2011 Dec 20. Annu Rev Biophys. 2012. PMID: 22224600 Review.
Cited by
-
Shark IgNAR: The Next Broad Application Antibody in Clinical Diagnoses and Tumor Therapies?Mar Drugs. 2023 Sep 16;21(9):496. doi: 10.3390/md21090496. Mar Drugs. 2023. PMID: 37755109 Free PMC article. Review.
-
Influence of Fluorination on Single-Molecule Unfolding and Rupture Pathways of a Mechanostable Protein Adhesion Complex.Nano Lett. 2020 Dec 9;20(12):8940-8950. doi: 10.1021/acs.nanolett.0c04178. Epub 2020 Nov 16. Nano Lett. 2020. PMID: 33191756 Free PMC article.
-
Deciphering the crystal structure of a novel nanobody against the NEIL1 DNA glycosylase.Acta Crystallogr D Struct Biol. 2024 Feb 1;80(Pt 2):137-146. doi: 10.1107/S205979832400038X. Epub 2024 Jan 30. Acta Crystallogr D Struct Biol. 2024. PMID: 38289715 Free PMC article.
-
Principles and Design of Molecular Tools for Sensing and Perturbing Cell Surface Receptor Activity.Chem Rev. 2025 Mar 12;125(5):2665-2702. doi: 10.1021/acs.chemrev.4c00582. Epub 2025 Feb 25. Chem Rev. 2025. PMID: 39999110 Review.
-
NbThermo: a new thermostability database for nanobodies.Database (Oxford). 2023 Apr 12;2023:baad021. doi: 10.1093/database/baad021. Database (Oxford). 2023. PMID: 37042467 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

