A Francisella novicida Mutant, Lacking the Soluble Lytic Transglycosylase Slt, Exhibits Defects in Both Growth and Virulence
- PMID: 31258523
- PMCID: PMC6587636
- DOI: 10.3389/fmicb.2019.01343
A Francisella novicida Mutant, Lacking the Soluble Lytic Transglycosylase Slt, Exhibits Defects in Both Growth and Virulence
Abstract
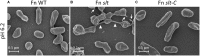
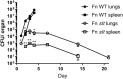
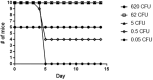
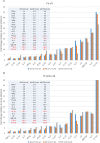
Francisella tularensis is the causative agent of tularemia and has gained recent interest as it poses a significant biothreat risk. F. novicida is commonly used as a laboratory surrogate for tularemia research due to genetic similarity and susceptibility of mice to infection. Currently, there is no FDA-approved tularemia vaccine, and identifying therapeutic targets remains a critical gap in strategies for combating this pathogen. Here, we investigate the soluble lytic transglycosylase or Slt in F. novicida, which belongs to a class of peptidoglycan-modifying enzymes known to be involved in cell division. We assess the role of Slt in biology and virulence of the organism as well as the vaccine potential of the slt mutant. We show that the F. novicida slt mutant has a significant growth defect in acidic pH conditions. Further microscopic analysis revealed significantly altered cell morphology compared to wild-type, including larger cell size, extensive membrane protrusions, and cell clumping and fusion, which was partially restored by growth in neutral pH or genetic complementation. Viability of the mutant was also significantly decreased during growth in acidic medium, but not at neutral pH. Furthermore, the slt mutant exhibited significant attenuation in a murine model of intranasal infection and virulence could be restored by genetic complementation. Moreover, we could protect mice using the slt mutant as a live vaccine strain against challenge with the parent strain; however, we were not able to protect against challenge with the fully virulent F. tularensis Schu S4 strain. These studies demonstrate a critical role for the Slt enzyme in maintaining proper cell division and morphology in acidic conditions, as well as replication and virulence in vivo. Our results suggest that although the current vaccination strategy with F. novicida slt mutant would not protect against Schu S4 challenges, the Slt enzyme could be an ideal target for future therapeutic development.
Keywords: Francisella; Francisella novicida; cell division; cell morphology; lytic transglycosylase; peptidoglycan (PG); tularemia; virulence.
Figures








References
-
- Ben Nasr A., Haithcoat J., Masterson J. E., Gunn J. S., Eaves-Pyles T., Klimpel G. R. (2006). Critical role for serum opsonins and complement receptors CR3 (CD11b/CD18) and CR4 (CD11c/CD18) in phagocytosis of Francisella tularensis by human dendritic cells (DC): uptake of Francisella leads to activation of immature DC and intracellular survival of the bacteria. J. Leukoc. Biol. 80 774–786. 10.1189/jlb.1205755 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

