The efficacy and safety of rifaximin-α: a 2-year observational study of overt hepatic encephalopathy
- PMID: 31258622
- PMCID: PMC6591657
- DOI: 10.1177/1756284819858256
The efficacy and safety of rifaximin-α: a 2-year observational study of overt hepatic encephalopathy
Abstract
Background: After 5 years since the registration of rifaximin-α as a secondary prophylaxis for overt hepatic encephalopathy (HE) in the Netherlands, we aimed to evaluate the use of hospital resources and safety of rifaximin-α treatment in a real-world setting.
Methods: We carried out prospective identification of all patients using rifaximin-α for overt HE. We assessed hospital resource use, bacterial infections, and adverse events during 6-month episodes before and after rifaximin-α initiation.
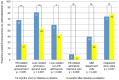
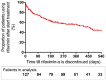
Results: During 26 months we included 127 patients [71.7% male; median age 60.8 years (interquartile range: 56.2-66.1); median model for end-stage liver disease (MELD) score 15.0 (interquartile range: 12.1-20.4); 98% using lactulose treatment]. When comparing the first 6 months after rifaximin-α initiation with the prior 6 months, HE-related hospital admissions decreased (0.86 to 0.41 admissions/patient; p < 0.001), as well as the mean length of stay (8.85 to 3.79 bed days/admission; p < 0.001). No significant differences were found regarding HE-related intensive care unit admissions (0.09 to 0.06 admission/patient; p = 0.253), stay on the intensive care unit (0.43 to 0.57 bed days/admission; p = 0.661), emergency department visits (0.66 to 0.51 visits/patient; p = 0.220), outpatient clinic visits (2.49 to 3.30 bed visits/patient; p = 0.240), or bacterial infections (0.41 to 0.35 infections/patient; p = 0.523). Adverse events were recorded in 2.4% of patients.
Conclusions: The addition of rifaximin-α to lactulose treatment was associated with a significant reduction in the number and length of HE-related hospitalizations for overt HE. Rifaximin-α treatment was well tolerated.
Keywords: efficacy; end-stage liver disease; healthcare utilization; hepatic encephalopathy; rifaximin-α; safety.
Conflict of interest statement
Conflict of interest statement: The authors have no conflict of interest. Sponsors did not actively participate in content development but reviewed the manuscript for scientific accuracy.
Figures

References
-
- Sherlock S, Dooley JS, Lok ASF, et al. Sherlock’s diseases of the liver and biliary system. Hoboken, NJ, USA: Wiley-Blackwell, 2002.
-
- Amodio P, Del Piccolo F, Petteno E, et al. Prevalence and prognostic value of quantified electroencephalogram (EEG) alterations in cirrhotic patients. J Hepatol 2001; 35: 37–45. - PubMed
-
- Bustamante J, Rimola A, Ventura PJ, et al. Prognostic significance of hepatic encephalopathy in patients with cirrhosis. J Hepatol 1999; 30: 890–895. - PubMed
-
- Jepsen P, Ott P, Andersen PK, et al. Clinical course of alcoholic liver cirrhosis: a Danish population-based cohort study. Hepatology 2010; 51: 1675–1682. - PubMed
-
- Arguedas MR, DeLawrence TG, McGuire BM. Influence of hepatic encephalopathy on health-related quality of life in patients with cirrhosis. Dig Dis Sci 2003; 48: 1622–1626. - PubMed
LinkOut - more resources
Full Text Sources

