Comparisons between protocols and publications of case-control studies: analysis of potential causes of non-reproducibility and recommendations for enhancing the quality of personalization in healthcare
- PMID: 31258815
- PMCID: PMC6562015
- DOI: 10.1007/s13167-019-00165-2
Comparisons between protocols and publications of case-control studies: analysis of potential causes of non-reproducibility and recommendations for enhancing the quality of personalization in healthcare
Abstract
Background: Selective reporting of results in published case-control studies has been widely suspected, but little comprehensive information on selective reporting is available with regard to case-control studies. We aimed to evaluate the concordance of findings between publications and the protocols of case-control studies and to assess the level of selective reporting of results in case-control studies.
Methods: The databases of Embase, Medline, Scopus, and Web of Science were searched to identify case-control study protocols published between January 1, 1990 and December 31, 2017. The numbers and characteristics of predefined exposures (or factors) were extracted from the protocols. The reported and unreported factors were both collected from the published studies and protocols. The frequency of selective reporting of results were estimated by identifying the discrepancies of factors between the protocols and the published studies. Study sample size and the extent of selective reporting of factors were measured by a Spearman correlation analysis.
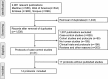
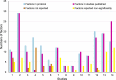
Results: Fourteen protocols with 24 published studies and 159 factors were identified, of which eight protocols (57.1%) had discrepancies between the publications and protocols. The prevalence of incomplete reporting in published case-control studies was 42.9% (6/14), with participant characteristics, anthropometric and laboratory measurement variables more likely to be unreported. A total of 16,835 cases and 56,049 controls were recruited in the 14 protocols of case-control studies (sample size ranges from 428 to 52,596 per study). Sample size had no statistical significance with selective reporting of results (P > 0.05).
Conclusion: The study protocols should be publicly available prior to the completion of case-control studies so that the potential bias can be assessed by the readers. Our findings highlight the need for investigators, peer reviewers, and readers to exercise increased awareness and scrutiny due to the undesirable practice of selective reporting of results in medical sciences causing the loss of potentially important information, thus impacting quality of personalized attitude in healthcare in the context of the predictive, preventive, and personalized medicine.
Keywords: Case-control study; Predictive preventive personalized medicine; Protocol; Publication bias; Quality of healthcare; Selective reporting of results.
Conflict of interest statement
Competing interestsThe authors declare that they have no competing interests.
Figures


Similar articles
-
Beyond the black stump: rapid reviews of health research issues affecting regional, rural and remote Australia.Med J Aust. 2020 Dec;213 Suppl 11:S3-S32.e1. doi: 10.5694/mja2.50881. Med J Aust. 2020. PMID: 33314144
-
Empirical evidence for selective reporting of outcomes in randomized trials: comparison of protocols to published articles.JAMA. 2004 May 26;291(20):2457-65. doi: 10.1001/jama.291.20.2457. JAMA. 2004. PMID: 15161896
-
Statistical controversies in clinical research: comparison of primary outcomes in protocols, public clinical-trial registries and publications: the example of oncology trials.Ann Oncol. 2017 Apr 1;28(4):688-695. doi: 10.1093/annonc/mdw682. Ann Oncol. 2017. PMID: 28011448
-
Comparison of conference abstracts and presentations with full-text articles in the health technology assessments of rapidly evolving technologies.Health Technol Assess. 2006 Feb;10(5):iii-iv, ix-145. doi: 10.3310/hta10050. Health Technol Assess. 2006. PMID: 16487455 Review.
-
Comparison between publicly accessible publications, registries, and protocols of phase III trials indicated persistence of selective outcome reporting.J Clin Epidemiol. 2017 Nov;91:87-94. doi: 10.1016/j.jclinepi.2017.07.010. Epub 2017 Jul 27. J Clin Epidemiol. 2017. PMID: 28757260 Review.
Cited by
-
Ten simple rules for successfully carrying out funded research projects.PLoS Comput Biol. 2024 Sep 19;20(9):e1012431. doi: 10.1371/journal.pcbi.1012431. eCollection 2024 Sep. PLoS Comput Biol. 2024. PMID: 39298382 Free PMC article. No abstract available.
References
LinkOut - more resources
Full Text Sources

