Prevalence of established and emerging biomarkers of immune checkpoint inhibitor response in advanced hepatocellular carcinoma
- PMID: 31258846
- PMCID: PMC6592287
- DOI: 10.18632/oncotarget.26998
Prevalence of established and emerging biomarkers of immune checkpoint inhibitor response in advanced hepatocellular carcinoma
Abstract
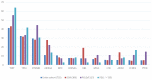
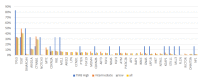
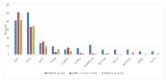
The clinical deployment of immune checkpoint inhibitors (ICIs) has created a tandem drive for the identification of biomarkers linked to benefit. Comprehensive genomic profiling was performed to evaluate the frequency of genomic biomarkers of ICI response in 755 patients with advanced hepatocellular carcinoma (HCC). Median age was 62 years' old, 73% were male, 46% had extrahepatic disease, 107 had documented hepatitis C, 96 had hepatitis B and 4 patients were coinfected. Median tumor mutation burden (TMB) was 4 mutations/Mb and only 6 tumors (0.8%) were TMB-high. Out of 542 cases assessed for microsatellite instability (MSI), one (0.2%) was MSI-high and TMB-high. Twenty-seven (4%) patients had POLE/D alterations. One patient had a pathogenic POLE R762W mutation but TMB was 4 mutations/Mb. Forty percent had DNA damage response gene alterations. In a small case series (N=17) exploring the relationship between biomarkers and ICI response, one patient (TMB 15 mutations/Mb, MSI-low) had a sustained complete response to nivolumab lasting > 2 years. Otherwise there were no significant genomic or TMB differences between responders, progressors, and those with stable disease. Overall, markers of genomic instability were infrequent in this cohort. Larger clinically annotated datasets are needed to explore genomic and non-genomic determinants of ICI response in HCC.
Keywords: biomarkers; hepatocellular carcinoma; immunotherapy; tumor mutation burden.
Conflict of interest statement
CONFLICTS OF INTEREST SMA, JSR, EAS, DF, JS, RM and SZM are employees of Foundation Medicine.
Figures
References
-
- El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling TH 3rd, Meyer T, Kang YK, Yeo W, et al. . Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017; 389:2492–502. 10.1016/S0140-6736(17)31046-2. - DOI - PMC - PubMed
-
- Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, Verslype C, Zagonel V, Fartoux L, Vogel A, Sarker D, Verset G, Chan SL, et al. , and KEYNOTE-224 investigators . Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018; 19:940–52. 10.1016/S1470-2045(18)30351-6. - DOI - PubMed
-
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, et al. . Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012; 366:2443–54. 10.1056/NEJMoa1200690. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

