Design and Implementation of Data Exchange Formats for Molecular Detection of Drug-Resistant Tuberculosis
- PMID: 31259025
- PMCID: PMC6568111
Design and Implementation of Data Exchange Formats for Molecular Detection of Drug-Resistant Tuberculosis
Abstract
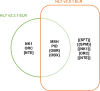
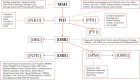
Drug-resistant tuberculosis (TB) remains a public health threat to the United States and worldwide control of TB. Rapid and reliable drug susceptibility testing (DST) is essential for aiding clinicians in selecting an optimal treatment regimen for TB patients and to prevent ongoing transmission. Growth-based DST results for culture-confirmed cases are routinely reported to the U.S. Centers for Disease Control and Prevention through the National TB Surveillance System (NTSS). However, the NTSS currently lacks the capacity and functionality to accept laboratory results from advanced molecular methods that detect mutations associated with drug resistance. The objective of this study is to design and implement novel comprehensive data exchange formats that utilize the Health Level Seven (HL7) version 2.5.1 messaging hierarchy to capture, store, and monitor molecular DST data, thereby, improving the quality of data, specifications and exchange formats within the NTSS as well as ensuring full reporting of drug-resistant TB.
Figures
References
-
- Curry International Tuberculosis Center and California Department of Public Health. Drug-Resistant Tuberculosis: A Survival Guide for Clinicians. (3rd ed) 2016
-
- Barnard M, Parsons L, Miotto P, Cirillo D, Feldmann K, Gutierrez C, Somoskovi A. Molecular Detection of Drug-Resistant Tuberculosis By Line Probe Assay - Laboratory Manual for Resource-Limited Settings. 2012
-
- Lin SY, Desmond EP. Molecular diagnosis of tuberculosis and drug resistance. Clin Lab Med. 2014;34(2):297–314. - PubMed
-
- Andrews JM. Determination of minimum inhibitory concentrations. J Antimicrob Chemother. 2001;48(Suppl 1(suppl_1)):5–16. - PubMed
LinkOut - more resources
Full Text Sources