In vivo multifunctional optical coherence tomography at the periphery of the lungs
- PMID: 31259075
- PMCID: PMC6583343
- DOI: 10.1364/BOE.10.003070
In vivo multifunctional optical coherence tomography at the periphery of the lungs
Abstract
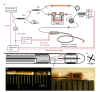
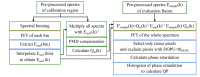
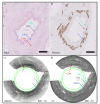
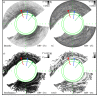
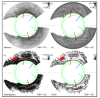
Remodeling of tissue, such as airway smooth muscle (ASM) and extracellular matrix, is considered a key feature of airways disease. No clinically accepted diagnostic method is currently available to assess airway remodeling or the effect of treatment modalities such as bronchial thermoplasty in asthma, other than invasive airway biopsies. Optical coherence tomography (OCT) generates cross-sectional, near-histological images of airway segments and enables identification and quantification of airway wall layers based on light scattering properties only. In this study, we used a custom motorized OCT probe that combines standard and polarization sensitive OCT (PS-OCT) to visualize birefringent tissue in vivo in the airway wall of a patient with severe asthma in a minimally invasive manner. We used optic axis uniformity (OAxU) to highlight the presence of uniformly arranged fiber-like tissue, helping visualizing the abundance of ASM and connective tissue structures. Attenuation coefficient images of the airways are presented for the first time, showing superior architectural contrast compared to standard OCT images. A novel segmentation algorithm was developed to detect the surface of the endoscope sheath and the surface of the tissue. PS-OCT is an innovative imaging technique that holds promise to assess airway remodeling including ASM and connective tissue in a minimally invasive, real-time manner.
Conflict of interest statement
The authors declare that there are no conflicts of interest related to this article.
Figures




References
LinkOut - more resources
Full Text Sources
