Proteomic identification of membrane-associated placental protein 4 (MP4) as perlecan and characterization of its placental expression in normal and pathologic pregnancies
- PMID: 31259093
- PMCID: PMC6589330
- DOI: 10.7717/peerj.6982
Proteomic identification of membrane-associated placental protein 4 (MP4) as perlecan and characterization of its placental expression in normal and pathologic pregnancies
Abstract
Background: More than 50 human placental proteins were isolated and physico-chemically characterized in the 70-80s by Hans Bohn and co-workers. Many of these proteins turned to have important role in placental functions and diagnostic significance in pregnancy complications. Among these proteins was membrane-associated placental protein 4 (MP4), for which identity or function has not been identified yet. Our aim was to analyze the sequence and placental expression of this protein in normal and complicated pregnancies including miscarriage, preeclampsia and HELLP syndrome.
Methods: Lyophilized MP4 protein and frozen healthy placental tissue were analyzed using HPLC-MS/MS. Placental tissue samples were obtained from women with elective termination of pregnancy (first trimester controls, n = 31), early pregnancy loss (EPL) (n = 13), early preeclampsia without HELLP syndrome (n = 7) and with HELLP syndrome (n = 8), late preeclampsia (n = 8), third trimester early controls (n = 5) and third trimester late controls (n = 9). Tissue microarrays were constructed from paraffin-embedded placentas (n = 81). Slides were immunostained with monoclonal perlecan antibody and evaluated using light microscopy and virtual microscopy. Perlecan was also analyzed for its expression in placentas from normal pregnancies using microarray data.
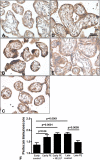
Results: Mass spectrometry-based proteomics of MP4 resulted in the identification of basement membrane-specific heparan sulfate proteoglycan core protein also known as perlecan. Immunohistochemistry showed cytoplasmic perlecan localization in syncytiotrophoblast and cytotrophoblasts of the villi. Perlecan immunoscore decreased with gestational age in the placenta. Perlecan immunoscores were higher in EPL compared to controls. Perlecan immunoscores were higher in early preeclampsia without and with HELLP syndrome and lower in late preeclampsia than in respective controls. Among patients with preeclampsia, placental perlecan expression positively correlated with maternal vascular malperfusion and negatively correlated with placental weight.
Conclusion: Our findings suggest that an increased placental perlecan expression may be associated with hypoxic ischaemic injury of the placenta in miscarriages and in early preeclampsia with or without HELLP syndrome.
Keywords: Miscarriage; Placenta; Preeclampsia; Pregnancy; Proteoglycan.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Proteomic identification of Placental Protein 1 (PP1), PP8, and PP22 and characterization of their placental expression in healthy pregnancies and in preeclampsia.Placenta. 2020 Sep 15;99:197-207. doi: 10.1016/j.placenta.2020.05.013. Epub 2020 Jun 22. Placenta. 2020. PMID: 32747003 Free PMC article.
-
Increased placental expression of Placental Protein 5 (PP5) / Tissue Factor Pathway Inhibitor-2 (TFPI-2) in women with preeclampsia and HELLP syndrome: Relevance to impaired trophoblast invasion?Placenta. 2019 Jan 15;76:30-39. doi: 10.1016/j.placenta.2019.01.011. Epub 2019 Jan 19. Placenta. 2019. PMID: 30803712
-
Changes of placental syndecan-1 expression in preeclampsia and HELLP syndrome.Virchows Arch. 2013 Sep;463(3):445-58. doi: 10.1007/s00428-013-1426-0. Epub 2013 Jun 27. Virchows Arch. 2013. PMID: 23807541 Free PMC article.
-
An integrated model of preeclampsia: a multifaceted syndrome of the maternal cardiovascular-placental-fetal array.Am J Obstet Gynecol. 2022 Feb;226(2S):S963-S972. doi: 10.1016/j.ajog.2020.10.023. Epub 2021 Mar 9. Am J Obstet Gynecol. 2022. PMID: 33712272 Review.
-
Why is placentation abnormal in preeclampsia?Am J Obstet Gynecol. 2015 Oct;213(4 Suppl):S115-22. doi: 10.1016/j.ajog.2015.08.042. Am J Obstet Gynecol. 2015. PMID: 26428489 Free PMC article. Review.
Cited by
-
Dysregulation of Placental Functions and Immune Pathways in Complete Hydatidiform Moles.Int J Mol Sci. 2019 Oct 10;20(20):4999. doi: 10.3390/ijms20204999. Int J Mol Sci. 2019. PMID: 31658584 Free PMC article.
-
Proteomic identification of Placental Protein 1 (PP1), PP8, and PP22 and characterization of their placental expression in healthy pregnancies and in preeclampsia.Placenta. 2020 Sep 15;99:197-207. doi: 10.1016/j.placenta.2020.05.013. Epub 2020 Jun 22. Placenta. 2020. PMID: 32747003 Free PMC article.
-
Review of Alterations in Perlecan-Associated Vascular Risk Factors in Dementia.Int J Mol Sci. 2020 Jan 20;21(2):679. doi: 10.3390/ijms21020679. Int J Mol Sci. 2020. PMID: 31968632 Free PMC article. Review.
-
Cathepsin D elevates the fibrocalcific activity in human aortic valve cells through the ERK1/2-Sox9 pathway.Front Cardiovasc Med. 2024 Sep 24;11:1410862. doi: 10.3389/fcvm.2024.1410862. eCollection 2024. Front Cardiovasc Med. 2024. PMID: 39380629 Free PMC article.
-
Proteoglycans: Systems-Level Insight into Their Expression in Healthy and Diseased Placentas.Int J Mol Sci. 2022 May 21;23(10):5798. doi: 10.3390/ijms23105798. Int J Mol Sci. 2022. PMID: 35628608 Free PMC article. Review.
References
-
- Balogh A, Pozsgay J, Matko J, Dong Z, Kim CJ, Varkonyi T, Sammar M, Rigo J, Jr, Meiri H, Romero R, Papp Z, Than NG. Placental protein 13 (PP13/galectin-13) undergoes lipid raft-associated subcellular redistribution in the syncytiotrophoblast in preterm preeclampsia and HELLP syndrome. American Journal of Obstetrics and Gynecology. 2011;205(2):156.e1–156.e14. doi: 10.1016/j.ajog.2011.03.023. - DOI - PMC - PubMed
-
- Bilban M, Haslinger P, Prast J, Klinglmuller F, Woelfel T, Haider S, Sachs A, Otterbein LE, Desoye G, Hiden U, Wagner O, Knofler M. Identification of novel trophoblast invasion-related genes: heme oxygenase-1 controls motility via peroxisome proliferator-activated receptor γ. Endocrinology. 2009;150(2):1000–1013. doi: 10.1210/en.2008-0456. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

