Mild Cognitive Impairment with a High Risk of Progression to Alzheimer's Disease Dementia (MCI-HR-AD): Effect of Souvenaid® Treatment on Cognition and 18F-FDG PET Scans
- PMID: 31259306
- PMCID: PMC6597964
- DOI: 10.3233/ADR-190109
Mild Cognitive Impairment with a High Risk of Progression to Alzheimer's Disease Dementia (MCI-HR-AD): Effect of Souvenaid® Treatment on Cognition and 18F-FDG PET Scans
Abstract
Background: Previous studies have shown that Souvenaid (medical food) can have benefits on memory, cognition, and function in early Alzheimer's disease (AD) and mild cognitive impairment (MCI).
Objective: Demonstrate that Souvenaid could improve or maintain cognition and has an effect on neurodegeneration biomarkers.
Methods: This cohort study was carried out from June 2015 through December 2016 in the Neurology Department, Infanta Cristina Hospital, Madrid, Spain. MCI-HR-AD were recruited using Petersen criteria, neuropsychology (NPS), and 18F-FDG PET scans to confirm the high risk of progression to dementia with one year of follow-up. Age, sex, vascular risk factors (VRF), and NPS values (Barcelona brief version) were analyzed. 18F-FDG PET scans were analyzed as a visual procedure. The study was approved by the Research Committee of ICH. Statistical analysis was made with SPSS 22.0 version.
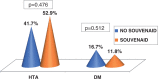
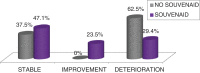
Results: Subjects included 43 MCI patients (58.5% women; mean age 69.78±7.89): 17 receiving Souvenaid® treatment (ST), 24 receiving no treatment (WT) and 2 who withdrew. No differences were seen in VRF, only hypercholesterolemia, and were less prevalent in the ST group (p = 0.002). The rate of progression to dementia was 48.8% (no differences between groups, p = 0.654). A second round of 18F-FDG PET scans showed a significance worsening of glucose metabolism in WT (p = 0.001) versus ST, in which it was low (p = 0.050). For NPS testing, there was a significant worsening in memory performance in the WT group (p = 0.011) and a stabilization in ST (p = 0.083), as well as in executive functions and attention (worsening in WT, p = 0.014). For the Subjective Changing Scale (SCS), caregivers indicated a stabilization/improvement in ST (p = 0.017).
Conclusion: Souvenaid had a significant effect on several cognitive domains, and on SCS in patients with MCI-HR-AD. Its intervention had an impact on preservation on 18F-FDG PET scans.
Keywords: 18F-FDG PET scans; Alzheimer’s disease; Souvenaid®; mild cognitive impairment; neuropsychology.
Conflict of interest statement
The authors have no conflict of interest to report.
Figures
Similar articles
-
Exploring effects of Souvenaid on cerebral glucose metabolism in Alzheimer's disease.Alzheimers Dement (N Y). 2019 Sep 27;5:492-500. doi: 10.1016/j.trci.2019.08.002. eCollection 2019. Alzheimers Dement (N Y). 2019. PMID: 31650005 Free PMC article.
-
Accelerated hypometabolism with disease progression associated with faster cognitive decline among amyloid positive patients.Front Neurosci. 2023 Apr 14;17:1151820. doi: 10.3389/fnins.2023.1151820. eCollection 2023. Front Neurosci. 2023. PMID: 37123373 Free PMC article.
-
The Association Between Brain Metabolic Biomarkers Using 18F-FDG and Cognition and Vascular Risk Factors, as well as Its Usefulness in the Diagnosis and Staging of Alzheimer's Disease.J Alzheimers Dis Rep. 2024 Sep 5;8(1):1229-1240. doi: 10.3233/ADR-240104. eCollection 2024. J Alzheimers Dis Rep. 2024. PMID: 39247877 Free PMC article.
-
Radiomics: a novel feature extraction method for brain neuron degeneration disease using 18F-FDG PET imaging and its implementation for Alzheimer's disease and mild cognitive impairment.Ther Adv Neurol Disord. 2019 Mar 29;12:1756286419838682. doi: 10.1177/1756286419838682. eCollection 2019. Ther Adv Neurol Disord. 2019. PMID: 30956687 Free PMC article.
-
18F-FDG PET for Prediction of Conversion to Alzheimer's Disease Dementia in People with Mild Cognitive Impairment: An Updated Systematic Review of Test Accuracy.J Alzheimers Dis. 2018;64(4):1175-1194. doi: 10.3233/JAD-171125. J Alzheimers Dis. 2018. PMID: 30010119 Free PMC article.
Cited by
-
Nutritional metabolism and cerebral bioenergetics in Alzheimer's disease and related dementias.Alzheimers Dement. 2023 Mar;19(3):1041-1066. doi: 10.1002/alz.12845. Epub 2022 Dec 8. Alzheimers Dement. 2023. PMID: 36479795 Free PMC article. Review.
-
Jidong cognitive impairment cohort study: objectives, design, and baseline screening.Neural Regen Res. 2020 Jun;15(6):1111-1119. doi: 10.4103/1673-5374.266070. Neural Regen Res. 2020. PMID: 31823892 Free PMC article.
-
Effects of preventive interventions on neuroimaging biomarkers in subjects at-risk to develop Alzheimer's disease: A systematic review.Front Aging Neurosci. 2022 Nov 24;14:1014559. doi: 10.3389/fnagi.2022.1014559. eCollection 2022. Front Aging Neurosci. 2022. PMID: 36506466 Free PMC article.
-
Efficacy of Souvenaid® Combined with Acetylcholinesterase Inhibitors in the Treatment of Mild Alzheimer's Disease.J Alzheimers Dis. 2023;91(4):1459-1469. doi: 10.3233/JAD-221003. J Alzheimers Dis. 2023. PMID: 36641676 Free PMC article.
-
Souvenaid in the management of mild cognitive impairment: an expert consensus opinion.Alzheimers Res Ther. 2019 Aug 17;11(1):73. doi: 10.1186/s13195-019-0528-6. Alzheimers Res Ther. 2019. PMID: 31421681 Free PMC article.
References
-
- Santana I, Farinha F, Freitas S, Rodrigues V, Carvalho Å (2015) [The epidemiology of dementia and Alzheimer disease in Portugal: Estimations of prevalence and treatment-costs] (in Portuguese). Med Port 28, 182–188. - PubMed
-
- Alzheimer’s Association (2015) 2015 Alzheimer’s disease facts and figures. Alzheimers Dement 11, 332–384. - PubMed
-
- Prince M, Albanese E, Prina M (2014) World Alzheimer Report 2014. Dementia and risk reduction: An analysis of protective and modifiable factors. Alzheimer’s Disease International, London, UK.
-
- Chiang K, Koo EH (2014) Emerging therapeutics for Alzheimer’s disease. Ann Rev Pharmacol Toxicol 54, 381–405. - PubMed
-
- Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Carrillo MC, Dickson DW, Duyckaerts C, Frosch MP, Masliah E, Mirra SS, Nelson PT, Schneider JA, Thal DR, Thies B, Trojanowski JQ, Vinters HV, Montine TJ (2012) National Institute on Aging—Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement 8, 1–13. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources