The KdpFABC complex - K+ transport against all odds
- PMID: 31259644
- PMCID: PMC6681667
- DOI: 10.1080/09687688.2019.1638977
The KdpFABC complex - K+ transport against all odds
Abstract
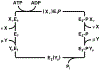
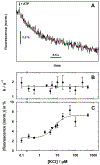
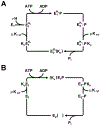
In bacteria, K+ is used to maintain cell volume and osmotic potential. Homeostasis normally involves a network of constitutively expressed transport systems, but in K+ deficient environments, the KdpFABC complex uses ATP to pump K+ into the cell. This complex appears to be a hybrid of two types of transporters, with KdpA descending from the superfamily of K+ transporters and KdpB belonging to the superfamily of P-type ATPases. Studies of enzymatic activity documented a catalytic cycle with hallmarks of classical P-type ATPases and studies of ion transport indicated that K+ import into the cytosol occurred in the second half of this cycle in conjunction with hydrolysis of an aspartyl phosphate intermediate. Atomic structures of the KdpFABC complex from X-ray crystallography and cryo-EM have recently revealed conformations before and after formation of this aspartyl phosphate that appear to contradict the functional studies. Specifically, structural comparisons with the archetypal P-type ATPase, SERCA, suggest that K+ transport occurs in the first half of the cycle, accompanying formation of the aspartyl phosphate. Further controversy has arisen regarding the path by which K+ crosses the membrane. The X-ray structure supports the conventional view that KdpA provides the conduit, whereas cryo-EM structures suggest that K+ moves from KdpA through a long, intramembrane tunnel to reach canonical ion binding sites in KdpB from which they are released to the cytosol. This review discusses evidence supporting these contradictory models and identifies key experiments needed to resolve discrepancies and produce a unified model for this fascinating mechanistic hybrid.
Keywords: Active transport; K homeostasis; P-type ATPase; Post-Albers cycle; protein structure; superfamily of K transporters; transport mechanism.
Conflict of interest statement
Disclosure Statement
The authors report no conflicts of interest.
Figures






Similar articles
-
Structural basis for potassium transport in prokaryotes by KdpFABC.Proc Natl Acad Sci U S A. 2021 Jul 20;118(29):e2105195118. doi: 10.1073/pnas.2105195118. Proc Natl Acad Sci U S A. 2021. PMID: 34272288 Free PMC article.
-
Crystal structure of the potassium-importing KdpFABC membrane complex.Nature. 2017 Jun 29;546(7660):681-685. doi: 10.1038/nature22970. Epub 2017 Jun 21. Nature. 2017. PMID: 28636601 Free PMC article.
-
Deciphering ion transport and ATPase coupling in the intersubunit tunnel of KdpFABC.Nat Commun. 2021 Aug 24;12(1):5098. doi: 10.1038/s41467-021-25242-x. Nat Commun. 2021. PMID: 34429416 Free PMC article.
-
The KdpFABC complex from Escherichia coli: a chimeric K+ transporter merging ion pumps with ion channels.Eur J Cell Biol. 2011 Sep;90(9):705-10. doi: 10.1016/j.ejcb.2011.04.011. Eur J Cell Biol. 2011. PMID: 21684627 Review.
-
The K+-translocating KdpFABC complex from Escherichia coli: a P-type ATPase with unique features.J Bioenerg Biomembr. 2007 Dec;39(5-6):397-402. doi: 10.1007/s10863-007-9111-0. J Bioenerg Biomembr. 2007. PMID: 18058005 Review.
Cited by
-
The PTSNtr-KdpDE-KdpFABC Pathway Contributes to Low Potassium Stress Adaptation and Competitive Nodulation of Sinorhizobium fredii.mBio. 2022 Jun 28;13(3):e0372121. doi: 10.1128/mbio.03721-21. Epub 2022 May 2. mBio. 2022. PMID: 35491828 Free PMC article.
-
Growth of soil ammonia-oxidizing archaea on air-exposed solid surface.ISME Commun. 2024 Oct 24;4(1):ycae129. doi: 10.1093/ismeco/ycae129. eCollection 2024 Jan. ISME Commun. 2024. PMID: 39544964 Free PMC article.
-
Partial Reactions of the Na,K-ATPase: Determination of Activation Energies and an Approach to Mechanism.J Membr Biol. 2020 Dec;253(6):631-645. doi: 10.1007/s00232-020-00153-y. Epub 2020 Nov 13. J Membr Biol. 2020. PMID: 33184678 Free PMC article.
-
Energy homeostasis is a conserved process: Evidence from Paracoccus denitrificans' response to acute changes in energy demand.PLoS One. 2021 Nov 8;16(11):e0259636. doi: 10.1371/journal.pone.0259636. eCollection 2021. PLoS One. 2021. PMID: 34748578 Free PMC article.
-
Serine phosphorylation regulates the P-type potassium pump KdpFABC.Elife. 2020 Sep 21;9:e55480. doi: 10.7554/eLife.55480. Elife. 2020. PMID: 32955430 Free PMC article.
References
-
- Ahnert F, Schmid R, Altendorf K, Greie JC. 2006. ATP binding properties of the soluble part of the KdpC subunit from the Escherichia coli K+-transporting KdpFABC P-type ATPase. Biochemistry 45:11038–11046. - PubMed
-
- Albers RW. 1967. Biochemical aspects of active transport. Ann Rev Biochem 36:727–756. - PubMed
-
- Altendorf K, Booth IR, Gralla J, Greie JC, Rosenthal AZ, Wood JM. 2009. Osmotic Stress. EcoSal Plus 3. - PubMed
-
- Altendorf K, Gaßel M, Puppe W, Mollenkamp T, Zeeck A, Boddien C et al. 1998. Structure and function of the Kdp-ATPase of Escherichia coli. Acta Physiol Scand Suppl 643:137–146. - PubMed
-
- Altendorf K, Siebers A, Epstein W. 1992. The KDP ATPase of Escherichia coli. Ann N Y Acad Sci 671:228–243. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
