The antagonism of folate receptor by dolutegravir: developmental toxicity reduction by supplemental folic acid
- PMID: 31259764
- PMCID: PMC6774845
- DOI: 10.1097/QAD.0000000000002289
The antagonism of folate receptor by dolutegravir: developmental toxicity reduction by supplemental folic acid
Erratum in
-
The antagonism of folate receptor by dolutegravir: developmental toxicity reduction by supplemental folic acid: Erratum.AIDS. 2021 Mar 15;35(4):709. doi: 10.1097/01.aids.0000733928.28423.53. AIDS. 2021. PMID: 33620879 No abstract available.
Abstract
Objective: Maternal folate (vitamin B9) status is the largest known modifier of neural tube defect risk, so we evaluated folate-related mechanisms of action for dolutegravir (DTG) developmental toxicity.
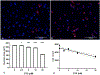
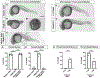
Design: Folate receptor 1 (FOLR1) was examined as a target for DTG developmental toxicity using protein and cellular interaction studies and an animal model.
Methods: FOLR1 competitive binding studies were used to test DTG for FOLR1 antagonism. Human placenta cell line studies were used to test interactions with DTG, folate, and cations. Zebrafish were selected as an animal model to examine DTG-induced developmental toxicity and rescue strategies.
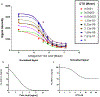
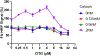
Results: FOLR1 binding studies indicate DTG is a noncompetitive FOLR1 antagonist at therapeutic concentrations. In-vitro testing indicates calcium (2 mmol/l) increases FOLR1-folate interactions and alters DTG-FOLR1-folate interactions and cytotoxicity. DTG does not inhibit downstream folate metabolism by dihydrofolate reductase. Early embryonic exposure to DTG is developmentally toxic in zebrafish, and supplemental folic acid can mitigate DTG developmental toxicity.
Conclusion: Folates and FOLR1 are established modifiers of risk for neural tube defects, and binding data indicates DTG is a partial antagonist of FOLR1. Supplemental folate can ameliorate increased developmental toxicity due to DTG in zebrafish. The results from these studies are expected to inform and guide future animal models and clinical studies of DTG-based antiretroviral therapy in women of childbearing age.
Figures
Comment in
-
Déjà vu all over again.AIDS. 2019 Nov 1;33(13):2097-2098. doi: 10.1097/QAD.0000000000002314. AIDS. 2019. PMID: 31577573 No abstract available.
-
Letter to the editor re: Cabrera et al., 2019 'The antagonism of folate receptor by dolutegravir: developmental toxicity reduction by supplemental folic acid'.AIDS. 2020 Jan 1;34(1):162-163. doi: 10.1097/QAD.0000000000002407. AIDS. 2020. PMID: 31789892 No abstract available.
References
-
- WHO. Updated recommendations on first-line and second-line antiretroviral regimens and post-exposure prophylaxis and recommendations on early infant diagnosis of HIV In: Supplement to the 2016 consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection Geneva: World Health Organization; 2018. pp. 82.
-
- Zash R, Holmes L, Makhema J, Diseko M, Jacobson DL, Mayondi G, et al. Surveillance for Neural Tube Defects following Antiretroviral Exposure from Conception In: 22nd International AIDS Conference. Amsterdam, Netherlands: NATAP; 2018.
-
- MRC. Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. MRC Vitamin Study Research Group. Lancet 1991; 338(8760):131–137. - PubMed
-
- Czeizel A, Dudas I. Prevention of the first occurrence of neural tube defects by periconceptional vitamin supplementation. New Engl J Med 1992; 327:1832–1835. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

