Generation of Tumor Organoids from Genetically Engineered Mouse Models of Prostate Cancer
- PMID: 31259888
- PMCID: PMC9014379
- DOI: 10.3791/59710
Generation of Tumor Organoids from Genetically Engineered Mouse Models of Prostate Cancer
Abstract
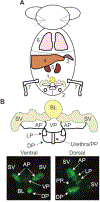
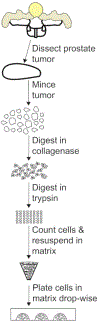
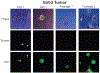
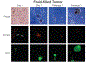
Methods based on homologous recombination to modify genes have significantly furthered biological research. Genetically engineered mouse models (GEMMs) are a rigorous method for studying mammalian development and disease. Our laboratory has developed several GEMMs of prostate cancer (PCa) that lack expression of one or multiple tumor suppressor genes using the site-specific Cre-loxP recombinase system and a prostate-specific promoter. In this article, we describe our method for necropsy of these PCa GEMMs, primarily focusing on dissection of mouse prostate tumors. New methods developed over the last decade have facilitated the culture of epithelial-derived cells to model organ systems in vitro in three dimensions. We also detail a 3D cell culture method to generate tumor organoids from mouse PCa GEMMs. Pre-clinical cancer research has been dominated by 2D cell culture and cell line-derived or patient-derived xenograft models. These methods lack tumor microenvironment, a limitation of using these techniques in pre-clinical studies. GEMMs are more physiologically-relevant for understanding tumorigenesis and cancer progression. Tumor organoid culture is an in vitro model system that recapitulates tumor architecture and cell lineage characteristics. In addition, 3D cell culture methods allow for growth of normal cells for comparison to tumor cell cultures, rarely possible using 2D cell culture techniques. In combination, use of GEMMs and 3D cell culture in pre-clinical studies has the potential to improve our understanding of cancer biology.
Figures

Similar articles
-
[Progress in prostate cancer study: 3D cell culture enables the ex vivo reproduction of tumor characteristics].Presse Med. 2017 Oct;46(10):954-965. doi: 10.1016/j.lpm.2017.06.014. Epub 2017 Sep 28. Presse Med. 2017. PMID: 28967525 French.
-
A synopsis of prostate organoid methodologies, applications, and limitations.Prostate. 2020 May;80(6):518-526. doi: 10.1002/pros.23966. Epub 2020 Feb 21. Prostate. 2020. PMID: 32084293 Review.
-
Protocols for Studies on Genetically Engineered Mouse Models in Prostate Cancer.Methods Mol Biol. 2018;1786:195-206. doi: 10.1007/978-1-4939-7845-8_11. Methods Mol Biol. 2018. PMID: 29786794
-
Genetically Engineered Mouse Models of Prostate Cancer in the Postgenomic Era.Cold Spring Harb Perspect Med. 2019 Feb 1;9(2):a030528. doi: 10.1101/cshperspect.a030528. Cold Spring Harb Perspect Med. 2019. PMID: 29661807 Free PMC article. Review.
-
Optimal, Large-Scale Propagation of Mouse Mammary Tumor Organoids.J Mammary Gland Biol Neoplasia. 2020 Dec;25(4):337-350. doi: 10.1007/s10911-020-09464-1. Epub 2020 Oct 26. J Mammary Gland Biol Neoplasia. 2020. PMID: 33106923 Free PMC article.
Cited by
-
Cellular and Molecular Mechanisms Underlying Prostate Cancer Development: Therapeutic Implications.Medicines (Basel). 2019 Jul 30;6(3):82. doi: 10.3390/medicines6030082. Medicines (Basel). 2019. PMID: 31366128 Free PMC article. Review.
-
Organoid of ovarian cancer: genomic analysis and drug screening.Clin Transl Oncol. 2020 Aug;22(8):1240-1251. doi: 10.1007/s12094-019-02276-8. Epub 2020 Jan 14. Clin Transl Oncol. 2020. PMID: 31939100 Free PMC article. Review.
-
A systematic review on the culture methods and applications of 3D tumoroids for cancer research and personalized medicine.Cell Oncol (Dordr). 2025 Feb;48(1):1-26. doi: 10.1007/s13402-024-00960-8. Epub 2024 May 28. Cell Oncol (Dordr). 2025. PMID: 38806997 Free PMC article.
References
-
- Capecchi MR Altering the genome by homologous recombination. Science. 244 (491 0), 1288–1292, (1989). - PubMed
-
- Aranda M et al. Altered directionality in the Cre-LoxP site-specific recombination pathway. Journal of Molecular Biology. 311 (3), 453–459, (2001). - PubMed
-
- Kuhn R, Schwenk F, Aguet M, & Rajewsky K Inducible gene targeting in mice. Science. 269 (5229), 1427–1429, (1995). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
