Real-Time, Semi-Automated Fluorescent Measurement of the Airway Surface Liquid pH of Primary Human Airway Epithelial Cells
- PMID: 31259916
- PMCID: PMC6748865
- DOI: 10.3791/59815
Real-Time, Semi-Automated Fluorescent Measurement of the Airway Surface Liquid pH of Primary Human Airway Epithelial Cells
Abstract
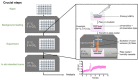
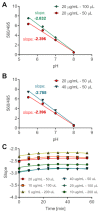
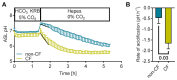
In recent years, the importance of mucosal surface pH in the airways has been highlighted by its ability to regulate airway surface liquid (ASL) hydration, mucus viscosity and activity of antimicrobial peptides, key parameters involved in innate defense of the lungs. This is of primary relevance in the field of chronic respiratory diseases such as cystic fibrosis (CF) where these parameters are dysregulated. While different groups have studied ASL pH both in vivo and in vitro, their methods report a relatively wide range of ASL pH values and even contradictory findings regarding any pH differences between non-CF and CF cells. Furthermore, their protocols do not always provide enough details in order to ensure reproducibility, most are low throughput and require expensive equipment or specialized knowledge to implement, making them difficult to establish in most labs. Here we describe a semi-automated fluorescent plate reader assay that enables the real-time measurement of ASL pH under thin film conditions that more closely resemble the in vivo situation. This technique allows for stable measurements for many hours from multiple airway cultures simultaneously and, importantly, dynamic changes in ASL pH in response to agonists and inhibitors can be monitored. To achieve this, the ASL of fully differentiated primary human airway epithelial cells (hAECs) are stained overnight with a pH-sensitive dye in order to allow for the reabsorption of the excess fluid to ensure thin film conditions. After fluorescence is monitored in the presence or absence of agonists, pH calibration is performed in situ to correct for volume and dye concentration. The method described provides the required controls to make stable and reproducible ASL pH measurements, which ultimately could be used as a drug discovery platform for personalized medicine, as well as adapted to other epithelial tissues and experimental conditions, such as inflammatory and/or host-pathogen models.
Figures


Similar articles
-
Molecular basis for pH-dependent mucosal dehydration in cystic fibrosis airways.Proc Natl Acad Sci U S A. 2013 Oct 1;110(40):15973-8. doi: 10.1073/pnas.1311999110. Epub 2013 Sep 16. Proc Natl Acad Sci U S A. 2013. PMID: 24043776 Free PMC article.
-
CFTR delivery to 25% of surface epithelial cells restores normal rates of mucus transport to human cystic fibrosis airway epithelium.PLoS Biol. 2009 Jul;7(7):e1000155. doi: 10.1371/journal.pbio.1000155. Epub 2009 Jul 21. PLoS Biol. 2009. PMID: 19621064 Free PMC article.
-
Airway surface liquid acidification initiates host defense abnormalities in Cystic Fibrosis.Sci Rep. 2019 Apr 24;9(1):6516. doi: 10.1038/s41598-019-42751-4. Sci Rep. 2019. PMID: 31019198 Free PMC article.
-
Effects of airway surface liquid pH on host defense in cystic fibrosis.Int J Biochem Cell Biol. 2014 Jul;52:124-9. doi: 10.1016/j.biocel.2014.02.009. Epub 2014 Feb 19. Int J Biochem Cell Biol. 2014. PMID: 24560894 Free PMC article. Review.
-
Inflammation as a Regulator of the Airway Surface Liquid pH in Cystic Fibrosis.Cells. 2023 Apr 7;12(8):1104. doi: 10.3390/cells12081104. Cells. 2023. PMID: 37190013 Free PMC article. Review.
Cited by
-
Reduced surface pH and upregulated AE2 anion exchange in SLC26A3-deleted polarized intestinal epithelial cells.Am J Physiol Cell Physiol. 2024 Mar 1;326(3):C829-C842. doi: 10.1152/ajpcell.00590.2023. Epub 2024 Jan 15. Am J Physiol Cell Physiol. 2024. PMID: 38223928 Free PMC article.
-
Inhibition of the sodium-dependent HCO3- transporter SLC4A4, produces a cystic fibrosis-like airway disease phenotype.Elife. 2022 May 30;11:e75871. doi: 10.7554/eLife.75871. Elife. 2022. PMID: 35635440 Free PMC article.
-
Pulmonary Delivery of Aerosolized Chloroquine and Hydroxychloroquine to Treat COVID-19: In Vitro Experimentation to Human Dosing Predictions.AAPS J. 2022 Feb 7;24(1):33. doi: 10.1208/s12248-021-00666-x. AAPS J. 2022. PMID: 35132508 Free PMC article.
-
Transport properties in CFTR-/- knockout piglets suggest normal airway surface liquid pH and enhanced amiloride-sensitive Na+ absorption.Pflugers Arch. 2020 Oct;472(10):1507-1519. doi: 10.1007/s00424-020-02440-y. Epub 2020 Jul 25. Pflugers Arch. 2020. PMID: 32712714 Free PMC article.
-
Dynamic regulation of airway surface liquid pH by TMEM16A and SLC26A4 in cystic fibrosis nasal epithelia with rare mutations.Proc Natl Acad Sci U S A. 2023 Nov 21;120(47):e2307551120. doi: 10.1073/pnas.2307551120. Epub 2023 Nov 15. Proc Natl Acad Sci U S A. 2023. PMID: 37967223 Free PMC article.
References
-
- Boucher RC. Human airway ion transport. Part one. American Journal of Respiratory and Critical Care Medicine. 1994;150(1):271–281. - PubMed
-
- Roomans GM, et al. Measurements of airway surface liquid height and mucus transport by fluorescence microscopy, and of ion composition by X-ray microanalysis. Journal of Cystic Fibrosis. 2004;3(Suppl 2):135–139. - PubMed
-
- Haq IJ, Gray MA, Garnett JP, Ward C, Brodlie M. Airway surface liquid homeostasis in cystic fibrosis: pathophysiology and therapeutic targets. Thorax. 2016;71(3):284–287. - PubMed
-
- Martin SL, Saint-Criq V, Hwang TC, Csanady L. Ion channels as targets to treat cystic fibrosis lung disease. Journal of Cystic Fibrosis. 2018;17(2S):S22–S27. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
