CD166 Engagement Augments Mouse and Human Hematopoietic Progenitor Function via Activation of Stemness and Cell Cycle Pathways
- PMID: 31260147
- PMCID: PMC9274619
- DOI: 10.1002/stem.3053
CD166 Engagement Augments Mouse and Human Hematopoietic Progenitor Function via Activation of Stemness and Cell Cycle Pathways
Abstract
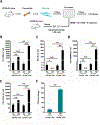
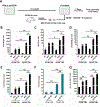
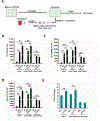
Hematopoietic stem (HSC) and progenitor (HPC) cells are regulated by interacting signals and cellular and noncellular elements of the hematopoietic niche. We previously showed that CD166 is a functional marker of murine and human HSC and of cellular components of the murine niche. Selection of murine CD166+ engrafting HSC enriched for marrow repopulating cells. Here, we demonstrate that CD166-CD166 homophilic interactions enhance generation of murine and human HPC in vitro and augment hematopoietic function of these cells. Interactions between cultured CD166+ Lineage- Sca-1+ c-Kit+ (LSK) cells and CD166+ osteoblasts (OBs) significantly enhanced the expansion of colony-forming units (CFUs). Interactions between CD166+ LSK cells and immobilized CD166 protein generated more CFU in short-term cultures than between these cells and bovine serum albumin (BSA) or in cultures initiated with CD166- LSK cells. Similar results were obtained when LSK cells from wildtype (WT) or CD166 knockout (KO) (CD166-/- ) mice were used with immobilized CD166. Human cord blood CD34+ cells expressing CD166 produced significantly higher numbers of CFUs following interaction with immobilized CD166 than their CD166- counterparts. These data demonstrate the positive effects of CD166 homophilic interactions involving CD166 on the surface of murine and human HPCs. Single-cell RNA-seq analysis of CD150+ CD48- (signaling lymphocyte activation molecule (SLAM)) LSK cells from WT and CD166-/- mice incubated with immobilized CD166 protein revealed that engagement of CD166 on these cells activates cytokine, growth factor and hormone signaling, epigenetic pathways, and other genes implicated in maintenance of stem cell pluripotency-related and mitochondria-related signaling pathways. These studies provide tangible evidence implicating CD166 engagement in the maintenance of stem/progenitor cell function. Stem Cells 2019;37:1319-1330.
Keywords: CD166; Hematopoietic stem and progenitor cells; Homophilic interaction; Single-cell RNA-seq analysis.
©AlphaMed Press 2019.
Conflict of interest statement
Conflicts of Interests
The authors declare no conflict of interest.
Figures


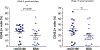
Similar articles
-
CD166 regulates human and murine hematopoietic stem cells and the hematopoietic niche.Blood. 2014 Jul 24;124(4):519-29. doi: 10.1182/blood-2014-03-565721. Epub 2014 Apr 16. Blood. 2014. PMID: 24740813 Free PMC article.
-
Impact of interactions of cellular components of the bone marrow microenvironment on hematopoietic stem and progenitor cell function.Blood. 2010 Apr 22;115(16):3239-48. doi: 10.1182/blood-2009-09-246173. Epub 2010 Feb 12. Blood. 2010. PMID: 20154218 Free PMC article.
-
Enrichment of hematopoietic stem cells with SLAM and LSK markers for the detection of hematopoietic stem cell function in normal and Trp53 null mice.Exp Hematol. 2008 Oct;36(10):1236-43. doi: 10.1016/j.exphem.2008.04.012. Epub 2008 Jun 17. Exp Hematol. 2008. PMID: 18562080 Free PMC article.
-
CD166 and regulation of hematopoiesis.Curr Opin Hematol. 2013 Jul;20(4):273-80. doi: 10.1097/MOH.0b013e32836060a9. Curr Opin Hematol. 2013. PMID: 23615053 Review.
-
Regulation of long-term repopulating hematopoietic stem cells by EPCR/PAR1 signaling.Ann N Y Acad Sci. 2016 Apr;1370(1):65-81. doi: 10.1111/nyas.13013. Epub 2016 Mar 1. Ann N Y Acad Sci. 2016. PMID: 26928241 Free PMC article. Review.
Cited by
-
Osteomacs promote maintenance of murine hematopoiesis through megakaryocyte-induced upregulation of Embigin and CD166.Stem Cell Reports. 2024 Apr 9;19(4):486-500. doi: 10.1016/j.stemcr.2024.02.004. Epub 2024 Mar 7. Stem Cell Reports. 2024. PMID: 38458190 Free PMC article.
-
Adipose-Derived Stem Cells from Fat Tissue of Breast Cancer Microenvironment Present Altered Adipogenic Differentiation Capabilities.Stem Cells Int. 2019 Aug 14;2019:1480314. doi: 10.1155/2019/1480314. eCollection 2019. Stem Cells Int. 2019. PMID: 31511776 Free PMC article.
-
Prostate cancer stem cells: an updated mini-review.J Cancer. 2024 Oct 28;15(20):6570-6576. doi: 10.7150/jca.100604. eCollection 2024. J Cancer. 2024. PMID: 39668824 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

