Beyond Charge Balance: Counter-Cations in Polyoxometalate Chemistry
- PMID: 31260159
- PMCID: PMC6972580
- DOI: 10.1002/anie.201905600
Beyond Charge Balance: Counter-Cations in Polyoxometalate Chemistry
Abstract
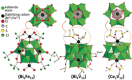
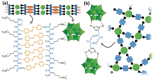
Polyoxometalates (POMs) are molecular metal-oxide anions applied in energy conversion and storage, manipulation of biomolecules, catalysis, as well as materials design and assembly. Although often overlooked, the interplay of intrinsically anionic POMs with organic and inorganic cations is crucial to control POM self-assembly, stabilization, solubility, and function. Beyond simple alkali metals and ammonium, chemically diverse cations including dendrimers, polyvalent metals, metal complexes, amphiphiles, and alkaloids allow tailoring properties for known applications, and those yet to be discovered. This review provides an overview of fundamental POM-cation interactions in solution, the resulting solid-state compounds, and behavior and properties that emerge from these POM-cation interactions. We will explore how application-inspired research has exploited cation-controlled design to discover new POM materials, which in turn has led to the quest for fundamental understanding of POM-cation interactions.
Keywords: Cations; Composites; Interactions; Polyoxometalates; Supramolecular Chemistry.
© 2019 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Special POM themed issue (Eds.: L. Cronin, A. Müller), Chem. Soc. Rev 2012, 41, 7325–7648.
-
- Special issue (Ed.: C. L. Hill), J. Mol. Catal. A 2007, 262, 2–6.
-
- Ji Y., Huang L., Hu J., Streb C., Song Y., Energy Environ. Sci. 2015, 8, 776–789.
-
- Shiddiq M., Komijani D., Duan Y., Gaita-Ariño A., Coronado E., Hill S., Nature 2016, 531, 348–351. - PubMed
-
- Herrmann S., Ritchie C., Streb C., Dalton Trans. 2015, 44, 7092–7104. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

