Procaspase-3 Overexpression in Cancer: A Paradoxical Observation with Therapeutic Potential
- PMID: 31260254
- PMCID: PMC6858495
- DOI: 10.1021/acschembio.9b00338
Procaspase-3 Overexpression in Cancer: A Paradoxical Observation with Therapeutic Potential
Abstract
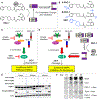
Many anticancer strategies rely on the promotion of apoptosis in cancer cells as a means to shrink tumors. Crucial for apoptotic function are executioner caspases, most notably caspase-3, that proteolyze a variety of proteins, inducing cell death. Paradoxically, overexpression of procaspase-3 (PC-3), the low-activity zymogen precursor to caspase-3, has been reported in a variety of cancer types. Until recently, this counterintuitive overexpression of a pro-apoptotic protein in cancer has been puzzling. Recent studies suggest subapoptotic caspase-3 activity may promote oncogenic transformation, a possible explanation for the enigmatic overexpression of PC-3. Herein, the overexpression of PC-3 in cancer and its mechanistic basis is reviewed; collectively, the data suggest the potential for exploitation of PC-3 overexpression with PC-3 activators as a targeted anticancer strategy.
Conflict of interest statement
The authors declare the following competing financial interest(s): The University of Illinois has filed patents on some of the technology described in this manuscript.
Figures

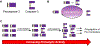
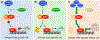
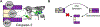


Similar articles
-
PAC-1 activates procaspase-3 in vitro through relief of zinc-mediated inhibition.J Mol Biol. 2009 Apr 24;388(1):144-58. doi: 10.1016/j.jmb.2009.03.003. Epub 2009 Mar 10. J Mol Biol. 2009. PMID: 19281821 Free PMC article.
-
Small-molecule activators of a proenzyme.Science. 2009 Nov 6;326(5954):853-8. doi: 10.1126/science.1177585. Science. 2009. PMID: 19892984 Free PMC article.
-
Small-molecule activation of procaspase-3 to caspase-3 as a personalized anticancer strategy.Nat Chem Biol. 2006 Oct;2(10):543-50. doi: 10.1038/nchembio814. Epub 2006 Aug 27. Nat Chem Biol. 2006. PMID: 16936720
-
Caspase-3: A primary target for natural and synthetic compounds for cancer therapy.Chem Biol Drug Des. 2021 Jul;98(1):144-165. doi: 10.1111/cbdd.13860. Epub 2021 May 20. Chem Biol Drug Des. 2021. PMID: 33963665 Review.
-
Targeting caspases in cancer therapeutics.Biol Chem. 2013 Jul;394(7):831-43. doi: 10.1515/hsz-2013-0128. Biol Chem. 2013. PMID: 23509217 Free PMC article. Review.
Cited by
-
Chrysophyllum cainito stem bark extract induces apoptosis in Human hepatocarcinoma HepG2 cells through ROS-mediated mitochondrial pathway.PeerJ. 2020 Oct 16;8:e10168. doi: 10.7717/peerj.10168. eCollection 2020. PeerJ. 2020. PMID: 33088629 Free PMC article.
-
Apoptosis - Fueling the oncogenic fire.FEBS J. 2021 Aug;288(15):4445-4463. doi: 10.1111/febs.15624. Epub 2020 Nov 25. FEBS J. 2021. PMID: 33179432 Free PMC article. Review.
-
Integrating network pharmacology, molecular docking, and bioinformatics to explore the mechanism of sparganii rhizoma in the treatment of laryngeal cancer.Mol Divers. 2025 Feb 26. doi: 10.1007/s11030-025-11142-5. Online ahead of print. Mol Divers. 2025. PMID: 40009149
-
Structure optimization of new tumor-selective Passerini α-acyloxy carboxamides as Caspase-3/7 activators.Sci Rep. 2022 Dec 27;12(1):22390. doi: 10.1038/s41598-022-26469-4. Sci Rep. 2022. PMID: 36575196 Free PMC article.
-
The combination of PAC-1 and entrectinib for the treatment of metastatic uveal melanoma.Melanoma Res. 2023 Dec 1;33(6):514-524. doi: 10.1097/CMR.0000000000000927. Epub 2023 Sep 22. Melanoma Res. 2023. PMID: 37738028 Free PMC article. Clinical Trial.
References
-
- Wyllie AH, Kerr JF, and Currie AR (1980) Cell death: the significance of apoptosis. Int. Rev. Cytol 68, 251–306. - PubMed
-
- Tsujimoto Y, Cossman J, Jaffe E, and Croce CM (1985) Involvement of the bcl-2 gene in human follicular lymphoma. Science 228, 1440–1443. - PubMed
-
- Vaux DL, Cory S, and Adams JM (1988) Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature 335, 440–442. - PubMed
-
- Hanahan D, and Weinberg RA (2000) The hallmarks of cancer. Cell 100, 57–70. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

