Uncoordinated maturation of developing and regenerating postnatal mammalian vestibular hair cells
- PMID: 31260439
- PMCID: PMC6602158
- DOI: 10.1371/journal.pbio.3000326
Uncoordinated maturation of developing and regenerating postnatal mammalian vestibular hair cells
Abstract
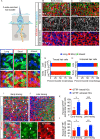
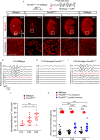
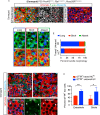
Sensory hair cells are mechanoreceptors required for hearing and balance functions. From embryonic development, hair cells acquire apical stereociliary bundles for mechanosensation, basolateral ion channels that shape receptor potential, and synaptic contacts for conveying information centrally. These key maturation steps are sequential and presumed coupled; however, whether hair cells emerging postnatally mature similarly is unknown. Here, we show that in vivo postnatally generated and regenerated hair cells in the utricle, a vestibular organ detecting linear acceleration, acquired some mature somatic features but hair bundles appeared nonfunctional and short. The utricle consists of two hair cell subtypes with distinct morphological, electrophysiological and synaptic features. In both the undamaged and damaged utricle, fate-mapping and electrophysiology experiments showed that Plp1+ supporting cells took on type II hair cell properties based on molecular markers, basolateral conductances and synaptic properties yet stereociliary bundles were absent, or small and nonfunctional. By contrast, Lgr5+ supporting cells regenerated hair cells with type I and II properties, representing a distinct hair cell precursor subtype. Lastly, direct physiological measurements showed that utricular function abolished by damage was partially regained during regeneration. Together, our data reveal a previously unrecognized aberrant maturation program for hair cells generated and regenerated postnatally and may have broad implications for inner ear regenerative therapies.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures









Similar articles
-
The Differentiation Status of Hair Cells That Regenerate Naturally in the Vestibular Inner Ear of the Adult Mouse.J Neurosci. 2021 Sep 15;41(37):7779-7796. doi: 10.1523/JNEUROSCI.3127-20.2021. Epub 2021 Jul 23. J Neurosci. 2021. PMID: 34301830 Free PMC article.
-
Spontaneous hair cell regeneration in the mouse utricle following gentamicin ototoxicity.Hear Res. 2009 Jan;247(1):17-26. doi: 10.1016/j.heares.2008.08.010. Epub 2008 Sep 7. Hear Res. 2009. PMID: 18809482 Free PMC article.
-
ADAM10 and γ-secretase regulate sensory regeneration in the avian vestibular organs.Dev Biol. 2017 Aug 1;428(1):39-51. doi: 10.1016/j.ydbio.2017.05.014. Epub 2017 May 17. Dev Biol. 2017. PMID: 28526588 Free PMC article.
-
Comparative biology of the amniote vestibular utricle.Hear Res. 2024 Jul;448:109035. doi: 10.1016/j.heares.2024.109035. Epub 2024 May 19. Hear Res. 2024. PMID: 38763033 Review.
-
A balance of form and function: planar polarity and development of the vestibular maculae.Semin Cell Dev Biol. 2013 May;24(5):490-8. doi: 10.1016/j.semcdb.2013.03.001. Epub 2013 Mar 15. Semin Cell Dev Biol. 2013. PMID: 23507521 Free PMC article. Review.
Cited by
-
Effects of cochlear hair cell ablation on spatial learning/memory.Sci Rep. 2020 Nov 26;10(1):20687. doi: 10.1038/s41598-020-77803-7. Sci Rep. 2020. PMID: 33244175 Free PMC article.
-
Pharmacological regeneration of sensory hair cells restores afferent innervation and vestibular function.J Clin Invest. 2024 Sep 24;134(22):e181201. doi: 10.1172/JCI181201. J Clin Invest. 2024. PMID: 39316439 Free PMC article.
-
Vestibular Deficits in Deafness: Clinical Presentation, Animal Modeling, and Treatment Solutions.Front Neurol. 2022 Apr 4;13:816534. doi: 10.3389/fneur.2022.816534. eCollection 2022. Front Neurol. 2022. PMID: 35444606 Free PMC article. Review.
-
Sox2 interacts with Atoh1 and Huwe1 loci to regulate Atoh1 transcription and stability during hair cell differentiation.PLoS Genet. 2025 Jan 30;21(1):e1011573. doi: 10.1371/journal.pgen.1011573. eCollection 2025 Jan. PLoS Genet. 2025. PMID: 39883720 Free PMC article.
-
Single-cell transcriptomic atlas reveals increased regeneration in diseased human inner ear balance organs.Nat Commun. 2024 Jun 6;15(1):4833. doi: 10.1038/s41467-024-48491-y. Nat Commun. 2024. PMID: 38844821 Free PMC article.
References
-
- Forge A, Li L, Corwin JT, Nevill G Ultrastructural evidence for hair cell regeneration in the mammalian inner ear. Science, (1993); 259: 1616–1619. - PubMed
-
- Forge A, Li L, Nevill G Hair cell recovery in the vestibular sensory epithelia of mature guinea pigs. J Comp Neurol, (1998); 397: 69–88. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

