Model-based assessment of public health impact and cost-effectiveness of dengue vaccination following screening for prior exposure
- PMID: 31260441
- PMCID: PMC6625736
- DOI: 10.1371/journal.pntd.0007482
Model-based assessment of public health impact and cost-effectiveness of dengue vaccination following screening for prior exposure
Abstract
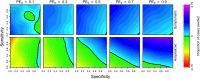
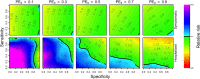
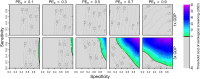
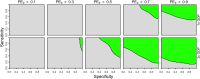
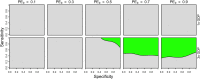
The tetravalent dengue vaccine CYD-TDV (Dengvaxia) is the first licensed vaccine against dengue, but recent findings indicate an elevated risk of severe disease among vaccinees without prior dengue virus (DENV) exposure. The World Health Organization currently recommends CYD-TDV only for individuals with serological confirmation of past DENV exposure. Our objective was to evaluate the potential health impact and cost-effectiveness of vaccination following serological screening. To do so, we used an agent-based model to simulate DENV transmission with and without vaccination over a 10-year timeframe. Across a range of values for the proportion of vaccinees with prior DENV exposure, we projected the proportion of symptomatic and hospitalized cases averted as a function of the sensitivity and specificity of serological screening. Scenarios about the cost-effectiveness of screening and vaccination were chosen to be representative of Brazil and the Philippines. We found that public health impact depended primarily on sensitivity in high-transmission settings and on specificity in low-transmission settings. Cost-effectiveness could be achievable from the perspective of a public payer provided that sensitivity and the value of a disability-adjusted life-year were both high, but only in high-transmission settings. Requirements for reducing relative risk and achieving cost-effectiveness from an individual perspective were more restricted, due to the fact that those who test negative pay for screening but receive no benefit. Our results predict that cost-effectiveness could be achieved only in high-transmission areas of dengue-endemic countries with a relatively high per capita GDP, such as Panamá (13,680 USD), Brazil (8,649 USD), México (8,201 USD), or Thailand (5,807 USD). In conclusion, vaccination with CYD-TDV following serological screening could have a positive impact in some high-transmission settings, provided that screening is highly specific (to minimize individual harm), at least moderately sensitive (to maximize population benefit), and sufficiently inexpensive (depending on the setting).
Conflict of interest statement
GE and TAP have received research support from GlaxoSmithKline for unrelated work on dengue vaccines. AWS is a consultant on vaccines for arboviral diseases for the World Health Organization (WHO). The author alone is responsible for the views expressed in this publication and they do not necessarily represent the decisions or policies of WHO. The authors declare no conflicts of interest.
Figures

Similar articles
-
Cost-effectiveness of dengue vaccination in Puerto Rico.PLoS Negl Trop Dis. 2021 Jul 26;15(7):e0009606. doi: 10.1371/journal.pntd.0009606. eCollection 2021 Jul. PLoS Negl Trop Dis. 2021. PMID: 34310614 Free PMC article.
-
The Long-Term Safety, Public Health Impact, and Cost-Effectiveness of Routine Vaccination with a Recombinant, Live-Attenuated Dengue Vaccine (Dengvaxia): A Model Comparison Study.PLoS Med. 2016 Nov 29;13(11):e1002181. doi: 10.1371/journal.pmed.1002181. eCollection 2016 Nov. PLoS Med. 2016. PMID: 27898668 Free PMC article.
-
Public health impact and cost-effectiveness of implementing a 'pre-vaccination screening' strategy with the dengue vaccine in Puerto Rico.Vaccine. 2022 Nov 28;40(50):7343-7351. doi: 10.1016/j.vaccine.2022.10.071. Epub 2022 Nov 5. Vaccine. 2022. PMID: 36347720
-
[Analysis of the evidence on the efficacy and safety of CYD-TDV dengue vaccine and its potential licensing and implementation through Mexico's Universal Vaccination Program].Salud Publica Mex. 2016 Jan-Feb;58(1):71-83. Salud Publica Mex. 2016. PMID: 26879510 Review. Spanish.
-
Updated recommendations of the International Dengue Initiative expert group for CYD-TDV vaccine implementation in Latin America.Vaccine. 2019 Oct 8;37(43):6291-6298. doi: 10.1016/j.vaccine.2019.09.010. Epub 2019 Sep 9. Vaccine. 2019. PMID: 31515144 Review.
Cited by
-
Cost-effectiveness and budget impact analyses of dengue vaccination in Indonesia.PLoS Negl Trop Dis. 2021 Aug 12;15(8):e0009664. doi: 10.1371/journal.pntd.0009664. eCollection 2021 Aug. PLoS Negl Trop Dis. 2021. PMID: 34383764 Free PMC article.
-
IgG Antibody Responses to the Aedes albopictus 34k2 Salivary Protein as Novel Candidate Marker of Human Exposure to the Tiger Mosquito.Front Cell Infect Microbiol. 2020 Jul 29;10:377. doi: 10.3389/fcimb.2020.00377. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32850479 Free PMC article.
-
Pandemic-associated mobility restrictions could cause increases in dengue virus transmission.PLoS Negl Trop Dis. 2021 Aug 9;15(8):e0009603. doi: 10.1371/journal.pntd.0009603. eCollection 2021 Aug. PLoS Negl Trop Dis. 2021. PMID: 34370734 Free PMC article.
-
Correlation between reported dengue illness history and seropositivity in rural Thailand.PLoS Negl Trop Dis. 2021 Jun 15;15(6):e0009459. doi: 10.1371/journal.pntd.0009459. eCollection 2021 Jun. PLoS Negl Trop Dis. 2021. PMID: 34129599 Free PMC article.
-
Risk factors for infection with chikungunya and Zika viruses in southern Puerto Rico: A community-based cross-sectional seroprevalence survey.PLoS Negl Trop Dis. 2022 Jun 13;16(6):e0010416. doi: 10.1371/journal.pntd.0010416. eCollection 2022 Jun. PLoS Negl Trop Dis. 2022. PMID: 35696355 Free PMC article.
References
-
- Capeding MR, Tran NH, Hadinegoro SRS, Ismail HIHJM, Chotpitayasunondh T, Chua MN, et al. Clinical efficacy and safety of a novel tetravalent dengue vaccine in healthy children in Asia: a phase 3, randomised, observer-masked, placebo-controlled trial. Lancet. 2014;384: 1358–1365. 10.1016/S0140-6736(14)61060-6 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

