Ablation of RhoA impairs Th17 cell differentiation and alleviates house dust mite-triggered allergic airway inflammation
- PMID: 31260596
- PMCID: PMC7747217
- DOI: 10.1002/JLB.3A0119-025RRR
Ablation of RhoA impairs Th17 cell differentiation and alleviates house dust mite-triggered allergic airway inflammation
Abstract
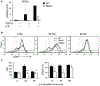
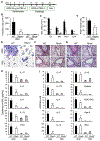
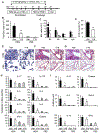
Asthma is a heterogeneous chronic airway inflammation in which Th2 and Th17 cells are key players in its pathogenesis. We have reported that RhoA of Rho GTPases orchestrated glycolysis for Th2 cell differentiation and allergic airway inflammation by the use of a conditional RhoA-deficient mouse line. However, the role of RhoA in Th17 cells remains to be elucidated. In this study, we investigated the effects of RhoA deficiency on Th17 cells in the context of ex vivo cell culture systems and an in vivo house dust mites (HDM)-induced allergic airway inflammation. We found that RhoA deficiency inhibited Th17 differentiation and effector cytokine secretion, which was associated with the downregulations of Stat3 and Rorγt, key Th17 transcription factors. Furthermore, loss of RhoA markedly suppressed Th17 and neutrophil-involved airway inflammation induced by HDM in mice. The infiltrating inflammatory cells in the lungs and bronchoalveolar lavage (BAL) fluids were dramatically reduced in conditional RhoA-deficient mice. Th17 as well as Th2 effector cytokines were suppressed in the airways at both protein and mRNA levels. Interestingly, Y16, a specific RhoA inhibitor, was able to recapitulate the most phenotypes of RhoA genetic deletion in Th17 differentiation and allergic airway inflammation. Our data demonstrate that RhoA is a key regulator of Th17 cell differentiation and function. RhoA might serve as a potential novel therapeutic target for asthma and other inflammatory disorders.
Keywords: RhoA; Th17; Y16; allergic airway inflammation; house dust mite.
©2019 Society for Leukocyte Biology.
Conflict of interest statement
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

