Circadian period 2: a missing beneficial factor in sickle cell disease by lowering pulmonary inflammation, iron overload, and mortality
- PMID: 31260634
- PMCID: PMC6988849
- DOI: 10.1096/fj.201900246RR
Circadian period 2: a missing beneficial factor in sickle cell disease by lowering pulmonary inflammation, iron overload, and mortality
Abstract
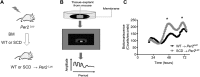
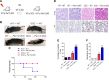
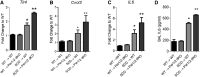
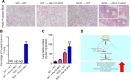
The circadian clock is important for cellular and organ function. However, its function in sickle cell disease (SCD), a life-threatening hemolytic disorder, remains unknown. Here, we performed an unbiased microarray screen, which revealed significantly altered expression of circadian rhythmic genes, inflammatory response genes, and iron metabolic genes in SCD Berkeley transgenic mouse lungs compared with controls. Given the vital role of period 2 (Per2) in the core clock and the unrecognized role of Per2 in SCD, we transplanted the bone marrow (BM) of SCD mice to Per2Luciferase mice, which revealed that Per2 expression was up-regulated in SCD mouse lung. Next, we transplanted the BM of SCD mice to period 1 (Per1)/Per2 double deficient [Per1/Per2 double knockout (dKO)] and wild-type mice, respectively. We discovered that Per1/Per2 dKO mice transplanted with SCD BM (SCD → Per1/Per2 dKO) displayed severe irradiation sensitivity and were more susceptible to an early death. Although we observed an increase of peripheral inflammatory cells, we did not detect differences in erythrocyte sickling. However, there was further lung damage due to elevated pulmonary congestion, inflammatory cell infiltration, iron overload, and secretion of IL-6 in lavage fluid. Overall, we demonstrate that Per1/Per2 is beneficial to counteract elevated systemic inflammation, lung tissue inflammation, and iron overload in SCD.-Adebiyi, M. G., Zhao, Z., Ye, Y., Manalo, J., Hong, Y., Lee, C. C., Xian, W., McKeon, F., Culp-Hill, R., D' Alessandro, A., Kellems, R. E., Yoo, S.-H., Han, L., Xia, Y. Circadian period 2: a missing beneficial factor in sickle cell disease by lowering pulmonary inflammation, iron overload, and mortality.
Keywords: circadian clock; heme-iron metabolism; lung.
Conflict of interest statement
The authors thank all the supporting authors, members of Y.X.’s laboratory for expertise, and the core facility at the University of Houston for providing the expertise needed to perform the microarray studies. Studies were performed with support of U.S. National Institutes of Health, National Heart, Lung, and Blood Institute Grants HL114457 (to Y.X.), HL113574 (to Y.X.), HL137990 (to Y.X.), and HL136969 (to Y.X.). The authors declare no conflicts of interest.
Figures

References
-
- Hassell K. L. (2010) Population estimates of sickle cell disease in the U.S. Am. J. Prev. Med. 38 (4 Suppl), S512–S521 - PubMed
-
- Steiner C. A., Miller J. L. (2006) Sickle cell disease patients in U.S. hospitals, 2004: statistical brief #21. In Healthcare Cost and Utilization Project (HCUP) Statistical Briefs, Agency for Healthcare Research and Quality (US), Rockville, MD, USA - PubMed
-
- Rees D. C., Williams T. N., Gladwin M. T. (2010) Sickle-cell disease. Lancet 376, 2018–2031 - PubMed
-
- Rees D. C., Gibson J. S. (2012) Biomarkers in sickle cell disease. Br. J. Haematol. 156, 433–445 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

