Distinct Genome Replication and Transcription Strategies within the Growing Filovirus Family
- PMID: 31260690
- PMCID: PMC6879820
- DOI: 10.1016/j.jmb.2019.06.029
Distinct Genome Replication and Transcription Strategies within the Growing Filovirus Family
Abstract
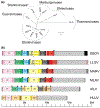
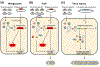
Research on filoviruses has historically focused on the highly pathogenic ebola- and marburgviruses. Indeed, until recently, these were the only two genera in the filovirus family. Recent advances in sequencing technologies have facilitated the discovery of not only a new ebolavirus, but also three new filovirus genera and a sixth proposed genus. While two of these new genera are similar to the ebola- and marburgviruses, the other two, discovered in saltwater fishes, are considerably more diverse. Nonetheless, these viruses retain a number of key features of the other filoviruses. Here, we review the key characteristics of filovirus replication and transcription, highlighting similarities and differences between the viruses. In particular, we focus on key regulatory elements in the genomes, replication and transcription strategies, and the conservation of protein domains and functions among the viruses. In addition, using computational analyses, we were able to identify potential homology and functions for some of the genes of the novel filoviruses with previously unknown functions. Although none of the newly discovered filoviruses have yet been isolated, initial studies of some of these viruses using minigenome systems have yielded insights into their mechanisms of replication and transcription. In general, the Cuevavirus and proposed Dianlovirus genera appear to follow the transcription and replication strategies employed by the ebola- and marburgviruses, respectively. While our knowledge of the fish filoviruses is currently limited to sequence analysis, the lack of certain conserved motifs and even entire genes necessitates that they have evolved distinct mechanisms of replication and transcription.
Keywords: Ebola virus; Marburg virus; filoviruses; genome replication and transcription; nonsegmented negative sense RNA viruses.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declarations of interest: none
Figures







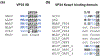
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

