The Role of SIRT1 on DNA Damage Response and Epigenetic Alterations in Cancer
- PMID: 31261609
- PMCID: PMC6651129
- DOI: 10.3390/ijms20133153
The Role of SIRT1 on DNA Damage Response and Epigenetic Alterations in Cancer
Abstract
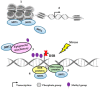
Sirtuin-1 (SIRT1) is a class-III histone deacetylase (HDAC), an NAD+-dependent enzyme deeply involved in gene regulation, genome stability maintenance, apoptosis, autophagy, senescence, proliferation, aging, and tumorigenesis. It also has a key role in the epigenetic regulation of tissue homeostasis and many diseases by deacetylating both histone and non-histone targets. Different studies have shown ambiguous implications of SIRT1 as both a tumor suppressor and tumor promoter. However, this contradictory role seems to be determined by the cell type and SIRT1 localization. SIRT1 upregulation has already been demonstrated in some cancer cells, such as acute myeloid leukemia (AML) and primary colon, prostate, melanoma, and non-melanoma skin cancers, while SIRT1 downregulation was described in breast cancer and hepatic cell carcinomas. Even though new functions of SIRT1 have been characterized, the underlying mechanisms that define its precise role on DNA damage and repair and their contribution to cancer development remains underexplored. Here, we discuss the recent findings on the interplay among SIRT1, oxidative stress, and DNA repair machinery and its impact on normal and cancer cells.
Keywords: DNA damage/repair; SIRT1; cancer development; epigenetics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

