Storage-Dependent Generation of Potent Anti-ZIKV Activity in Human Breast Milk
- PMID: 31261806
- PMCID: PMC6669682
- DOI: 10.3390/v11070591
Storage-Dependent Generation of Potent Anti-ZIKV Activity in Human Breast Milk
Abstract
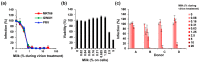
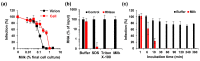
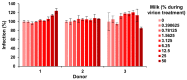
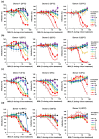
Zika virus (ZIKV) causes congenital neurologic birth defects, notably microcephaly, and has been associated with other serious complications in adults. The virus has been detected in human breast milk and possible transmissions via breastfeeding have been reported. Breast milk is rich in nutrients and bio-active substances that might directly affect viral infectivity. Thus, here, we analyzed the effect of human breast milk on ZIKV infection. We observed that fresh human breast milk had no effect on ZIKV, but found that upon storage, milk effectively suppressed infection. The antiviral activity is present in the fat-containing cream fraction of milk and results in the destruction of the structural integrity of viral particles, thereby abrogating infectivity. The release of the factor is time dependent but varies with donors and incubation temperatures. The viral titer of milk that was spiked with ZIKV decreased considerably upon storage at 37 °C for 8 h, was lost entirely after 2 days of 4 °C storage, but was not affected at -20 °C. This suggests that cold storage of milk inactivates ZIKV and that the antiviral factor in milk may also be generated upon breastfeeding and limit this transmission route of ZIKV.
Keywords: breast milk; breastfeeding; transmission; zika virus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- World Health Organization Zika Virus Situation Report—5th February 2016. [(accessed on 16 May 2019)]; Available online: http://www.who.int/emergencies/zika-virus/situation-report/5-february-20...
-
- World Health Organization Zika Situation Report—10 March 2017. [(accessed on 16 May 2019)]; Available online: https://www.who.int/emergencies/zika-virus/situation-report/10-march-201...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

