Ddx4+ Oogonial Stem Cells in Postmenopausal Women's Ovaries: A Controversial, Undefined Role
- PMID: 31261822
- PMCID: PMC6678385
- DOI: 10.3390/cells8070650
Ddx4+ Oogonial Stem Cells in Postmenopausal Women's Ovaries: A Controversial, Undefined Role
Abstract
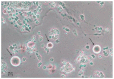
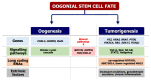
Recent studies support the existence of oogonial stem cells (OSCs) in the ovarian cortex of different mammals, including women.These cells are characterized by small size, membrane expression of DEAD(Asp-Glu-Ala-Asp)-box polypeptide-4 (Ddx4), and stemness properties (such as self-renewal and clonal expansion) as well as the ability to differentiate in vitro into oocyte-like cells. However, the discovery of OSCs contrasts with the popular theory that there is a numerically defined oocyte pool for female fertility which undergoes exhaustion with menopause. Indeed, in the ovarian cortex of postmenopausal women OSCs have been detected that possess both viability and capability to differentiate into oocytes, which is similar to those observed in younger patients. The pathophysiological role of this cell population in aged women is still debated since OSCs, under appropriate stimuli, differentiate into somatic cells, and the occurrence of Ddx4+ cells in ovarian tumor samples also suggests their potential involvement in carcinogenesis. Although further investigation into these observations is needed to clarify OSC function in ovary physiology, clinical investigators and researchers studying female infertility are presently focusing on OSCs as a novel opportunity to restore ovarian reserve in both young women undergoing early ovarian failure and cancer survivors experiencing iatrogenic menopause.
Keywords: Ddx4; infertility; menopause; oogonial stem cells; ovarian failure.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Zuckerman S. The number of oocytes in the mature ovary. Recent Prog. Horm. Res. 1951;6:63–108.
-
- Stimpfel M., Skutella T., Cvjeticanin B., Meznaric M., Dovc P., Novakovic S., Cerkovnik P., Vrtacnik-Bokal E., Virant-Klun I. Isolation, characterization and differentiation of cells expressing pluripotent/multipotent markers from adult human ovaries. Cell Tissue Res. 2013;354:593–607. doi: 10.1007/s00441-013-1677-8. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

