Telmisartan Inhibits Cell Proliferation and Tumor Growth of Esophageal Squamous Cell Carcinoma by Inducing S-Phase Arrest In Vitro and In Vivo
- PMID: 31261874
- PMCID: PMC6651359
- DOI: 10.3390/ijms20133197
Telmisartan Inhibits Cell Proliferation and Tumor Growth of Esophageal Squamous Cell Carcinoma by Inducing S-Phase Arrest In Vitro and In Vivo
Abstract
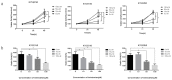
Esophageal squamous cell carcinoma (ESCC) is the most common primary esophageal malignancy. Telmisartan, an angiotensin II type 1 (AT1) receptor blocker (ARB) and a widely used antihypertensive, has been shown to inhibit proliferation of various cancer types. This study evaluated the effects of telmisartan on human ESCC cell proliferation in vitro and in vivo and sought to identify the microRNAs (miRNAs) involved in these antitumor effects. We examined the effects of telmisartan on three human ESCC cell lines (KYSE150, KYSE180, and KYSE850). Telmisartan inhibited proliferation of these three cell lines by inducing S-phase arrest, which was accompanied by decreased expression of cyclin A2, cyclin-dependent kinase 2, and other cell cycle-related proteins. Additionally, telmisartan reduced levels of phosphorylated ErbB3 and thrombospondin-1 in KYSE180 cells. Furthermore, expression of miRNAs was remarkably altered by telmisartan in vitro. Telmisartan also inhibited tumor growth in vivo in a xenograft mouse model. In conclusion, telmisartan inhibited cell proliferation and tumor growth in ESCC cells by inducing cell-cycle arrest.
Keywords: angiotensin II type 1 receptor blocker; cell cycle arrest; cyclin; esophageal squamous cell carcinoma; telmisartan.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- World Cancer Research Fund International Oesophageal Cancer Report 2016. [(accessed on 10 June 2019)]; Available online: http://www.wcrf.org/dietandcancer/oesophageal-cancer.
-
- Furihata M., Ishikawa T., Inoue A., Yoshikawa C., Sonobe H., Ohtsuki Y., Araki K., Ogoshi S. Determination of the prognostic significance of unscheduled cyclin A overexpression in patients with esophageal squamous cell carcinoma. Clin. Cancer Res. 1996;2:1781–1785. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

